In our daily lives we deal all the time with various types of radiation, each of which has a type of wavelength and, consequently, an energy different from the other.
The radiations that are most clearly perceived are the colors, which are in the visible region. But as the electromagnetic spectrum below shows, there are also other types of radiation, and one of them, which is invisible to our eyes, is the infrared (IR) radiation.
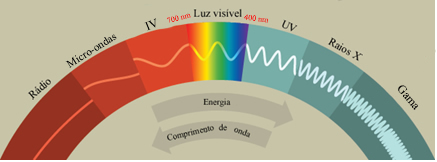
This type of radiation received its name because of its location in the electromagnetic spectrum and the way in which it was discovered, which was carried out by the English astronomer. William Herschel in 1800. He knew that a body's temperature is a measure of the thermal agitation of its particles and that each of the colors in the visible region had a different temperature. When he placed a mercury thermometer in the region of each of these colors, the result was that the temperature increased by the incidence of light, but it was faster the closer to the red end.
So he experimented with placing the thermometer bulb in a region that was after the red, which is a region where there is no color, that is, it is part of the invisible region of the spectrum, and he observed that in this region the warming was even greater. In this way, Herschel concluded that there must be some kind of radiation that our eye did not perceive and, since it was before the red band, he called it infrared radiation (meaning “below red”).
IR rays have çwavelength between 700 nm and 50,000 nm. Since the wavelength is inversely proportional to the energy, this type of radiation is of low energy, which is in the range of energy needed to make the atoms of a substance vibrate without causing a reaction, so it is non-radiation. ionizing.
Thanks to this, there is a branch, called infrared spectroscopy, that uses this type of radiation to identify a compound or investigate the composition of a sample, based on the fact that the Chemical bonds of substances have specific vibration frequencies, which correspond to the molecule's energy levels.
Do not stop now... There's more after the advertising ;)
This type of radiation is emitted by hot objects like the sun. Therefore, although they are not seen, they can be felt in the form of heat. 70% of the sun's rays that reach our planet manage to reach the Earth's surface, part of which is absorbed by it and the rest is reflected in the form of IR radiation. A portion of this radiation, in turn, is absorbed by clouds and CO2 of the atmosphere, creating a greenhouse effect that keeps the Earth warm and prevents large variations in temperature between day and night.
This type of radiation coming from the Sun does not cause great effects on the human organism, as its energy is low, its penetration power into the skin is also not great, acting mainly on the surface in the form of heat. However, in excess, it can cause burns.
Every body, including the human, emits infrared radiation. The higher the temperature, the greater the emission of these radiations, and human beings feel the presence of this type of radiation better when they are very intense, as they release heat. O iron it's the heater are examples of equipment that emit in the infrared range.
At infrared lamps use this radiation to activate blood circulation and reduce inflammatory processes. At infrared sensitive cameras they can show the hottest areas of the bodies, which are yellow and orange.

In addition, many devices used in everyday life work through IR radiation. For example, the Remote Control has a led which emits this radiation, which is then detected by a sensor in an electronic device such as a television. Other examples are the barcode readers and the mice that communicate with computers.

By Jennifer Fogaça
Graduated in Chemistry



