In the text "How do catalyst substances work?”, it has been shown that catalysts are able to increase the speed of reactions because they decrease the activation energy required for the reaction to occur. They do this by changing the reaction mechanism, combining with the reactants and forming an intermediate compound, which in turn becomes the products and catalyst.
One of the ways for this to happen is called Homogeneous catalysis, which is when the catalyst forms a single-phase system with the reactants.
This means that the reactants, products and catalyst all need to be in the same phase, that is, in the same physical state.
The study of this type of catalysis is important for science and industry, as several important reactions for production can be accelerated with small amounts of catalysts.
An example of homogeneous catalysis used in industry is an intermediate step in the manufacture of sulfuric acid (H2ONLY4(aq)), in which the formation of sulfur trioxide (SO3(g)) through the combustion reaction of sulfur dioxide (SO2(g)), shown below:
2 SO2(g) + O2(g) → 2 OS3(g)
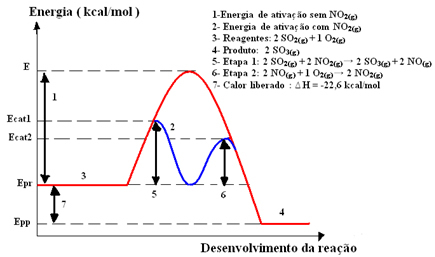
This reaction proceeds too slowly, so a catalyst, nitrogen dioxide (NO) is added.2(g)). This catalyst combines with sulfur dioxide to form an intermediate compound (activated complex), which is nitrogen monoxide (NO(g)).
Do not stop now... There's more after the advertising ;)
This intermediate compound then reacts with oxygen gas (O2(g)) for catalyst regeneration. See below how this occurs and observe how they are all in the gas phase, forming a homogeneous medium:
catalystcomplex activated
Step 1: 2 OS2(g) + 2 NO2(g)→ 2 OS3(g) + 2 NO(g)
Step 2: 2 NO(g)+ 1 O2(g) → 2 NO2 (g)
Global Reaction: 2 SO2(g) + O2(g) → 2 OS3(g)
Note that the catalyst only participates in the intermediate steps, but is not consumed. At the end of the reaction, he is fully recovered. The reaction with this mechanism made in two steps requires less activation energy to occur and, therefore, it proceeds more quickly.
Graphic representation:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Homogeneous Catalysis"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/catalise-homogenea.htm. Accessed on June 27, 2021.