Imagine that you add 10 g of table salt (sodium chloride - NaCl) to a glass of 100 g of water at 20ºC. After mixing, you see that the salt has completely dissolved, so you decide to add even more salt. At some point, you will no longer be able to dissolve the salt in that amount of water, and any added salt will sink to the bottom of the glass, no matter how hard you try to mix it.
When that happens, we say the solution is saturated and that the solubility coefficient. Therefore, we can define the solubility coefficient as follows:
“The solubility coefficient is the maximum amount of solute that is solubilized in a given amount of solvent, at a given temperature.”
The solubility coefficient of salt in water, for example, is equal to 36 g of NaCl/100 g of water at 20ºC. It is not possible to dissolve an extra gram of salt in this amount of water and at this temperature, as the solubility coefficient is specific for each substance. If we change the solute, for example, replacing table salt with NH4Cl, this has a solubility coefficient equal to 37.2 g in 100 g of water at 20°C.
Furthermore, the same substance has different solubilities in different solvents. While salt is soluble in water, it is practically insoluble in acetone or ethyl acetate (a solvent used to remove glazes).
Another point is that whenever the solubility coefficient of a solute in a given amount of solvent is mentioned, it is also necessary to indicate the temperature, as this is an interfering factor. For example, if we take 100 g of water at 20°C and add 40 g of salt, 36 g will solubilize and 4 g will form the precipitate. But if we take this solution to heating, we will see that the 4 g will dissolve as the temperature rises.
This shows us that the same solute dissolved in the same amount of solvent has different solubility coefficients as the temperature increases.
Do not stop now... There's more after the advertising ;)
See an example below:
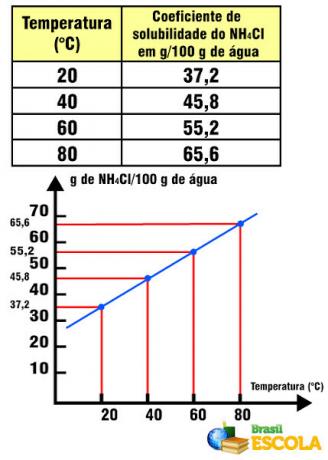
NH solubility coefficient4Cl in relation to temperature
Note that in this case, the solubility coefficient of NH4Cl increases with increasing temperature. This happens with most salts in water. However, there are situations in which the solubility coefficient decreases with increasing temperature, as in the case of Ce2(ONLY4)3. There are also cases in which there is not such a marked variability in the solubility coefficient, as occurs with table salt. See this in the text Graphs of solubility curves.
It may be that we can also, in certain specific situations, dissolve an amount of solute in the solvent greater than its solubility coefficient, thus obtaining the so-called supersaturated solution. For example, imagine that a solution formed with 100 g of water, at 20°C, and 40 g of table salt (with 36 g dissolved and 4 g precipitated), is heated until it reaches a temperature at which all the solute solubilize yourself. Then, this solution is left to rest so that it cools down until it reaches room temperature, which is close to 20ºC.
If there is no disturbance in the solution, the extra solute will remain dissolved, thus constituting a supersaturated solution. However, this type of solution is very unstable, and any sudden movement can cause the amount above the solubility coefficient for that temperature to crystallize. Thus, the solution that was supersaturated will become saturated with background body.
A last case is the unsaturated solution, which is when the amount of solute dissolved is less than the value of the solubility coefficient. An example is the dissolution of 10 g of NaCl in 100 g of water at 20°C.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Solubility coefficient"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/coeficiente-solubilidade.htm. Accessed on June 28, 2021.



