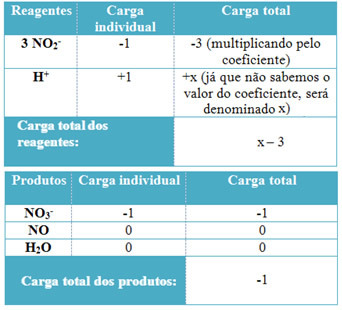
THE Electrochemistry is the field of research that studies the existing relationships between oxidation-reduction reactions and electrical current. This area is basically divided into two opposite branches:
- Batteries: this area studies the transformation of chemical energy into electrical energy. In these devices, redox reactions, in which electrons are transferred, produce electrical currents that are used to make certain pieces of equipment work. This is a spontaneous process.
- Electrolysis: studies how electrical energy is transformed into chemical energy. The passage of electrical current from a generator (such as a cell or battery) through a liquid system produces redox reactions. Since electrical current must be supplied, this process is not spontaneous.
In our society, the interactions between electricity and chemical transformations become increasingly important, as they provide a development of technology, discoveries of energy sources, issues related to Medicine, well-being and convenience for people and so on. Therefore, be sure to read the texts in this section and see how much Electrochemistry is essential to our lives.
Do not stop now... There's more after the advertising ;)
By Jennifer Fogaça
Graduated in Chemistry