One reversible reaction it occurs when it is possible to transform the products back into the reactants. This kind of reaction comes into chemical balance when the rate of development of the direct reaction, that is, the reaction with consumption of reagents and formation of products occurs at the same speed as the inverse reaction – formation of reactants and consumption of products.
These types of balanced reactions are seen a lot in everyday life. An example is what happens in caves, in the formation of so-called stalactites and stalagmites.
Groundwater is subject to high pressures, so it contains large amounts of carbon dioxide (CO2(g)) dissolved in it. When passing through soils that contain limestone (CaCO3(s)), this carbonate dissolves and caves are formed, according to the reaction below:
CaCO3(s) + CO2(g) + H2O(1) → Ca2+(here) + 2 HCO-3(aq)
However, carbonate and calcium ions can react, re-precipitating as calcium carbonate. This is demonstrated by the reaction:
Here2+(here) + 2 HCO-3(aq)→ CaCO3(s) + CO2(g) + H2O(1)
Do not stop now... There's more after the advertising ;)
As you may have noticed, one reaction is just the opposite of the other: reactants become products and products become reactants. So, we can represent this reaction in balance as follows:
CaCO3(s) + CO2(aq) + H2O(1) Here2+(here) + 2 HCO-3(aq)
Here2+(here) + 2 HCO-3(aq)
The slow and continuous disposition of carbonate from the mineralized drops from the roof of the caves run down, their water evaporates and there is CO release2. So the chemical balance is shifted in the opposite direction (of formation of reagents), hence the CaCO3(s) is formed, that is, the stalactites on the roof of the caves. When falling, the drop still has dissolved the carbonate, which is deposited on the cave floor, forming the stalagmites.
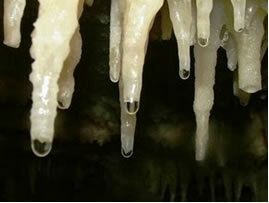
Water with limestone dripping for the formation of stalactites and stalagmites
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Chemical Balance in the Caves"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/equilibrio-quimico-nas-cavernas.htm. Accessed on June 28, 2021.


