THE auto-oxi-reduction or disproportionate reaction is a type of redox reaction in which the same chemical element undergoes oxidation and reduction. Let's look at two examples of this type of reaction and how to balance them using the redox method:
1st Example:
AT THE2- + H+ → NO3- + NO + H2O
- By calculating the oxidation numbers (NOX) of all atoms and ions involved in the reaction, it is possible to verify who oxidized and who reacted:
+3 -2 +1 +5 -2 +2 -2 +1 -2
AT THE2- + H+ → NO3- + NO + H2O
- Note that nitrogen was the species that both reacted and oxidized:
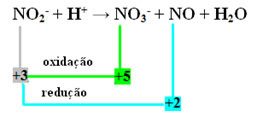
- To carry out the redox balancing of this reaction, we have to relate the NOX to the products, not the reagents:
AT THE3- =∆Nox = 5 - 3 = 2
NO=∆Nox = 3 - 2 = 1
- Inverting the ∆NOX by the coefficients, we have:
AT THE3- =∆NOX= 2 → 2 will be the coefficient of NO
NO=∆NOX= 1→ 1 will be the coefficient of NO3-
AT THE2- + H+ → 1 AT THE3- + 2 NO+H2O
- With this, we already know that there are 3 N in the product, so the coefficient of NO2- will be 3:
3 NO2- + H+ → 1 NO3- + 2 NO + H2O
- To determine the coefficients of H+ and from H2O, remember that the number of electrons received equals the same amount of electrons donated; thus, the reagent charge will be equal to the product charge. In this way, we can make the following scheme:
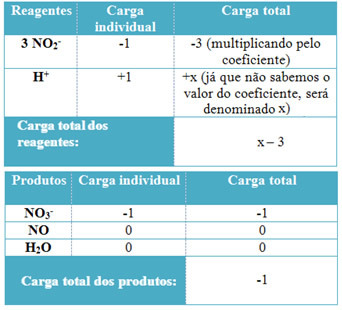
Do not stop now... There's more after the advertising ;)
Based on this information, we have that the total charge of the reagents is equal to x – 3 and the product is equal to -1. As stated, the charges of the two have to be equal. As we already have the total load of products, we can perform a simple calculation to know what the value of x will be:
x -3 = -1
x = -1 +3
x = 2
Thus, the coefficient of H+ is 2 and, consequently, that of H2The will be 1:
3 NO2- + 2 H+ → 1 NO3- + 2 NO + 1 H2O
2nd Example:
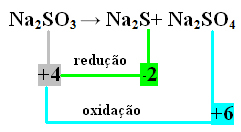
In this case, it was the S that underwent reduction and oxidation at the same time. Thus, as previously done, we can relate the NOX to the products and invert their values, assigning the coefficients to them:
At2 S=∆Nox = 4 – (-2) = 6 → 6 will be the coefficient of Na2 ONLY4
At2 ONLY4=∆Nox = 6 - 4 = 2 → 2 will be the coefficient of Na2 s
At2 ONLY3→ 2 At2 Y+ 6 At2 ONLY4
Since there are 8 sulfurs in the 2nd limb, the Na coefficient2 ONLY3 will be 8:
8 In2 ONLY3→ 2 In2 S + 6 In2 ONLY4
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Self-oxidizing reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-auto-oxirreducao.htm. Accessed on June 28, 2021.


