In the text “Alpha (α) emissions” an experiment carried out by Rutherford was mentioned, which consisted basically in placing a sample of radioactive material in a lead block. Through a hole in the block and an electromagnetic field, radioactive emissions were guided.
The French physicist Paul Ulrich Villard (1860-1934) repeated this experiment – in the same year that Rutherford performed it (1900) – and found that one of the radiations emitted it was not deflected by the electromagnetic field. This means that these emissions were not made up of particles, such as alpha (α) and beta (β) radiation, but were actually electromagnetic radiation.
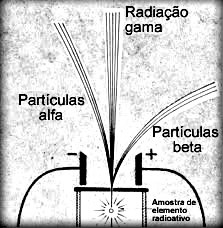
This electromagnetic radiation emitted by radioactive elements was called gamma radiation and represented by the Greek letter γ.
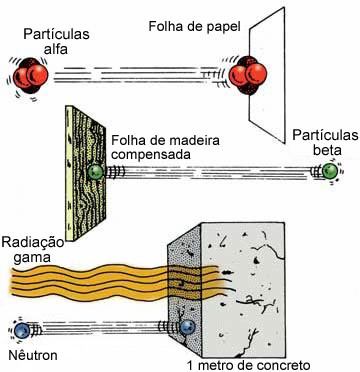
They are similar to X-rays, having no electrical charge and no mass. However, they are more energetic than X-rays, because their wavelength is much smaller, being between 0.1Å and 0.001Å. They are capable of traversing thousands of meters in air, sheets of paper, wooden boards, 15 cm of steel and are only held by lead plates or more than 5 cm of thick concrete walls.
Do not stop now... There's more after the advertising ;)
Furthermore, its high penetration power is also due to the fact that, as it has no electrical charge, it does not suffer interference from the electrons and protons of the atoms of the materials it passes through.
As a result, gamma emissions they can pass through a human body and cause irreparable damage. When it passes through matter, this radiation interacts with molecules, resulting in ions and free radicals, the latter of which are harmful to living cells. Some cells are more sensitive, such as those in the lymphatic tissue, those in the marrow, those in the intestinal mucous membranes, those in the gonads and those in the lens of the eyes.
See below its penetration power compared to alpha and beta radiation:
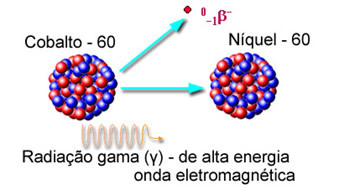
They are emitted by the nucleus immediately after the exit of the α or β particles. Therefore, even an alpha particle-emitting element can be dangerous, as it also emits γ rays.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Gamma emissions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/emissoes-gama.htm. Accessed on June 27, 2021.