The French chemist Joseph Louis Proust (1754-1826) began to carry out careful experiments relating the masses of the components of certain reactions.
For example, the elements that make up water are hydrogen and oxygen. Proust found that in this water-forming reaction, hydrogen always reacted with oxygen in a constant and definite ratio, which was 1:8, respectively. See how this happens below:

Notice that no matter how much mass of elements used, the proportion will always be the same.
Proust noted that this was not just the case with water, but with all other substances.
15.06 g of cupric sulfide (CuS) are formed, for example, by reacting 10.00 g of metallic copper (Cu) with 5.06 g of sulfur (S). So, if we double the amount of copper (which will go to 20.0 g), and if we want all the copper to react, it will also be necessary to double the amount of sulfur, to 10.12 g, with the total formation of 30.12 g of sulfide.
Now, if an amount is added that is not in proportion, the excess amount will be left over, it will not react. Note this below:
Do not stop now... There's more after the advertising ;)
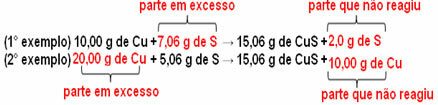
Notice that the reaction only takes place according to the defined proportion. Therefore, in 1799, Proust concluded that, when several substances combine to form a compound, this is always done in a defined mass relationship.
So he created a Law that is called Proust's Law, Law of Constant Proportions or Law of Defined Proportions, which is stated as follows:

This law and the Lavoisier's Law (Mass Conservation Law) are named Weight Laws, because they speak in masses of substances involved.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Proust's Law or Law of Constant Proportions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/lei-proust-ou-lei-das-proporcoes-constantes.htm. Accessed on June 27, 2021.


