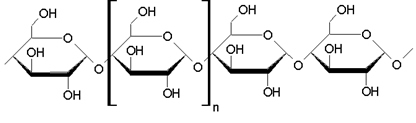
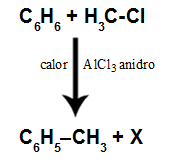
question 1
In a laboratory, a chemistry student needed to store some alkali metals (eg sodium, lithium and potassium) in containers without the presence of atmospheric air, as these metals react intensely with water in any physical state. Knowing that water is a polar solvent, in which of the following liquids would the student be able to store alkali metals without risk of explosion?
a) acetone
b) kerosene
c) methoxy-methane
d) ethanol
e) acetic acid
question 2
When comparing the boiling points of organic compounds, we take into account the forces that bind its molecules, which have a direct relationship with the polar or non-polar characteristic of the compound. So, comparing the compounds:
I) ethanol
II) ethanal
III) isopropyl isobutyl ether
IV) propane
Which ones are polar?
a) I and III
b) I and II
c) II and III
d) I and IV
e) III and IV
More questionsIn today's video we'll learn about hydrolysis! Paying attention to the word, you get an idea that it is a breakage caused by the action of water. Let's understand a little more about this process!
Durkheim, one of the founders of sociology, defined it as the science of social facts. Learn all about social facts in this video lesson by Professor João Gabriel.