Water is a substance that has numerous interesting properties that greatly benefit life, one of which is its superficial tension.
THE water surface tension is a result of hydrogen bonds, which are intermolecular forces caused by the attraction of hydrogens from certain water molecules (which are the positive poles (H+)) with the oxygens of neighboring molecules (which are the negative poles (O-)).
However, the attractive force of molecules on the surface of the water is different from the force that occurs between the molecules below the surface. This is because the latter are attracted to other water molecules in all directions: up, down, left, right, forward and backward. This means that they attract each other with equal strength.
As for the surface molecules, they do not have molecules above them, so their hydrogen bonds are restricted to molecules on the side and below. This inequality of surface attractions creates a force on these molecules and causes the liquid to contract, causing the called surface tension, which works as a thin layer, film, or as if it were a thin elastic membrane on the surface. from water.
Do not stop now... There's more after the advertising ;)
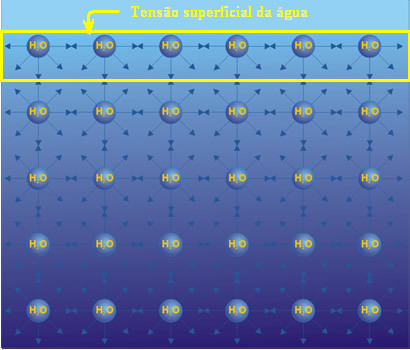
The surface tension of water is the highest of all liquids, equal to 7.2. 109 No. m-1.
This explains several phenomena. Among them, the main ones are:
- The spherical shape of water drops:

- Some insects can walk on water. Even in lakes, there are two communities of microorganisms: the neustons, which are bacteria, fungi and algae; and the pleustons, formed by superior plants and some small animals, such as larvae and crustaceans. These communities are sustained by the surface tension of the water.
- This phenomenon also explains why small objects, such as razor blades and clips (which are made of steel and therefore have an approximate density of 8 g/cm3), do not sink when placed horizontally over water.

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Surface Water Tension"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/tensao-superficial-agua.htm. Accessed on June 28, 2021.
Chemistry

Water pollution, physical aspects of water, chemical aspects of water, biological aspects of water, industrial waste, heavy metals, drinking water, organic matter, water turbidity, sewage.