When we cook food in our homes, we use cooking gas combustion. One of the things that we can see in the flame resulting from the combustion of this gas is that its color is normally a very light blue. However, in most combustion reactions, such as burning a candle, the flame is yellow.

Then the question arises:
"If every fire is the result of a combustion reaction, why do some flames have different colors?"
To understand how this happens, we have to understand what a combustion reaction is, and what substances are present in each of the reactions mentioned.
A combustion reaction occurs when a fuel (oxidizable material) is consumed by an oxidizer (a gaseous material containing oxygen) to generate thermal energy (heat).
Another important point we need to know about combustion reactions is that they can take place in a complete or incomplete. If there is enough oxygen to consume the fuel, the reaction will be complete and produce carbon dioxide (CO2) and water (H2O). Otherwise, combustion will be partial, incomplete, generating carbon monoxide (CO) and water; or carbon (C) and water.
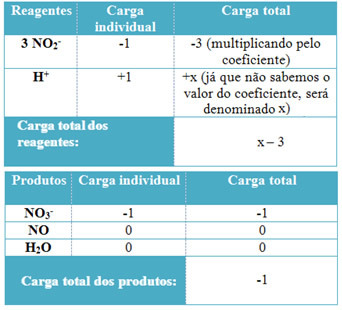
In both cases we are analyzing, the oxidizer is the oxygen present in the air. However, fuels are different. Cooking gas is actually liquefied petroleum gas (LPG), which is a mixture of hydrocarbons (alkanes), the main fuel being butane (C4H10). Thus, cooking gas is made up of alkane molecules that have only three or four carbon atoms, that is why little oxygen is needed for its combustion to take place completely. This reaction can be expressed as follows:
1C4H10(g) + 13/2 O2(g) → 4 CO2(g) + 5 hours2O(g), ∆H < 0
Do not stop now... There's more after the advertising ;)
In the case of candles, the paraffin is the fuel for the reaction, and it is made up of a mixture of alkanes with carbon atoms that range from 20 to 36. Thereby, it takes a lot more oxygen for this reaction to take place fully. See an example:
1C24H50(s) + 70/2 O2(g) → 25 CO2(g) + 25 H2O(g), ∆H < 0
In the air there is not enough oxygen to carry out this complete combustion, so it takes place incompletely, as shown below:
1C24H50(s) +49/2 O2(g) → 24 CO(g) + 25 H2O(g), ∆H < 0
1C24H50(s) +25/2 O2(g) → 24C(s) + 25 H2O(g), ∆H < 0
Incomplete reactions produce less energy than complete combustion. This explains the difference between the colors of the flames, as the yellow flame, characteristic of incomplete combustion, has lower energy. The blue flame is characteristic of a complete combustion, with greater energy.
This also explains the formation of soot by the candle flame (pictured below), which is carbon considered to be the product of incomplete combustion.
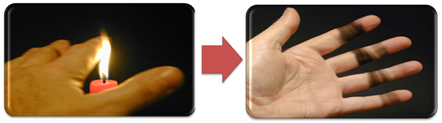
But why, in the Bunsen burner, is it possible to get yellow and blue flames when the fuel doesn't change?
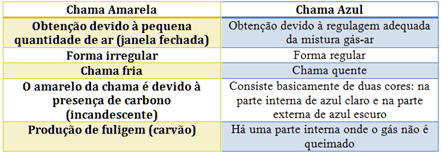
In the case of the Bunsen burner, this is achieved by regulating the inlet of gas and air. If the window is closed, causing the entry of a small amount of air, the flame obtained will be yellow, because it will have little oxygen to carry out complete combustion. If the regulation of the gas-air mixture is adequate, we get a blue flame.
See the characteristics of each in the table below:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Combustion and flames of different colors"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/combustao-chamas-cores-diferentes.htm. Accessed on June 28, 2021.