Rearrangement polymers are those in which at least one of their monomers undergoes rearrangements in its chemical structure as the polymerization reaction occurs.
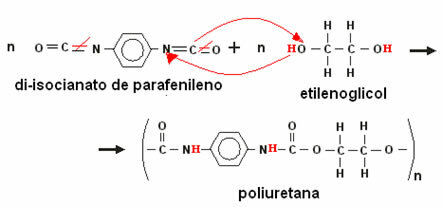
The most important example of this type of polymer is the polyurethane or polyurethane. Polyurethane is formed by the reaction between paraphenylene diisocyanate and ethylene glycol, as per the reaction below:
Do not stop now... There's more after the advertising ;)
This polymer is mainly used to give rise to rigid and flexible foams, when mixing their monomers with freon gas, which is a gas that tends to break off, expanding the polymer and forming the foam.
However, polyurethane is also used in the production of condoms, textile fibers, insulators, in car seats, carpets, shoes, etc.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Rearrangement Polymers"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/polimeros-rearranjo.htm. Accessed on June 28, 2021.