Consider the decay reaction of tritium, an isotope of hydrogen that has mass number three, with two neutrons and a proton in its nucleus. Below we can see that when emitting a beta particle (-10β), one of its neutrons turns into a proton and it becomes a helium atom:
13T → 23He + -10β
Note that over a period of exactly 12 years the mass of a tritium sample was reduced by half. This means that if we have a sample of this radioactive isotope equal to 10 mg, after 12 years this mass will reduce to 5 mg. After another 12 years, we will only have 2.5 mg and so on. Tritium will continue to emit radiation until it is completely gone.
The graph below represents this radioactive decay of tritium:

Studying the radioactive decay of other radioisotopes showed that the radiation intensity of all of them also halved in a regular period. For example, carbon-14 is halved in 5,730 years, iron-59 is reduced every 45 days, and technetium-99 is reduced every six hours.
Do not stop now... There's more after the advertising ;)
This shows us two important facts: (1) for each radioactive isotope, the time it is reduced by half is constant; (2) this time varies from one isotope to another.
With that, the concept of half-life emerged:

This concept is important for many purposes. For example, the decay time of carbon-14 is reused to determine the age of mummies and some fossils, as you can see in the text Carbon 14. Furthermore, the age of the Earth can be estimated through the radioactive decay of uranium-238. Knowing how long a radioisotope takes to disintegrate also helps in determining how long a given atomic waste should remain. isolated, as well as helping scientists to research the effects and applications of various radioisotopes in medicine, agriculture and foods.
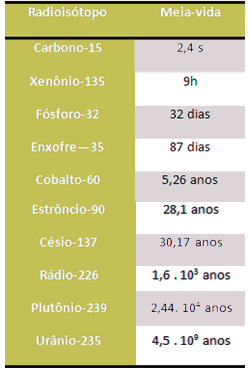
Half-life does not depend on initial sample amount, pressure or temperature. These periods can range from billions of years to fractions of seconds. See this in the list below:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Half-life or semi-disintegration period"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/meia-vida-ou-periodo-semidesintegracao.htm. Accessed on June 27, 2021.