Can you imagine if you went to a supermarket and found all the sections mixed up? Fruits on the same shelves as cleaning products, drinks mixed with personal care products, finally, it would really be a mess and it would be much harder to find the items on your shopping list, wouldn't it?!
This example shows us that separating similar items into specific groups makes our lives easier. This same idea applies to the study of chemical substances, as the separation into groups makes the study of these compounds easier. For example, the Inorganic chemistry have the inorganic functions, which are the groups acids, bases, salts and oxides.
Similarly, the Organic chemistry It has organic functions which are groups of organic compounds that have similar chemical properties., that is, given certain substances and specific conditions, the compounds belonging to the same organic function behave in a very similar way.
This similarity in chemical behavior is linked to the presence of the same. functional group
. We can define a functional group as a grouping of atoms that appears in the structure of the carbon chain and that is responsible for the similarity in the chemical behavior of a series of compounds.Below we present the main organic functions, the functional group that identifies each one, the naming rules according to IUPAC and examples of compounds belonging to these functions:
1- Organic Function: Hydrocarbons;
- Functional Group: It has only carbon and hydrogen atoms: C, H;
- Nomenclature: Prefix + infix + o;
- Examples: Methane, butane, ethene (ethylene) and ethine (acetylene).

The main source of hydrocarbons in nature is oil
2- Organic Function:alcohols;
- Functional Group: It has the hydroxyl linked to a saturated carbon:
oh
│
─ C ─
│
- Nomenclature: Prefix + infix + ol;
- Examples: Methanol, ethanol and propanol.

Alcohol gel and brandy have ethanol as the main constituent
3- Organic Function:Phenols;
- Functional Group: It has the hydroxyl (OH) linked to an unsaturated carbon of a benzene ring (aromatic nucleus):

Functional group of phenols
- Nomenclature: location of the OH + hydroxy + aromatic name;
- Examples: benzenel and 1-hydroxy-2-methylbenzene.
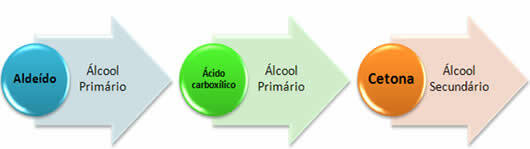
4- Organic Function: Aldehydes;
- Functional Group: It has the carbonyl linked to a hydrogen:
O
//
─C
H
- Nomenclature: Prefix + infix + al;
- Examples: Methanal (in aqueous solution is formaldehyde) and ethanal (acetaldehyde).
5- Organic Function: Ketones;
- Functional Group: It has the carbonyl between two carbons:
O
║
C C ─ C
- Nomenclature:Prefix + infix + ona;
Do not stop now... There's more after the advertising ;)
- Example: Propanone (acetone).

The acetone used to remove nail polish is propanone
6- Organic Function: carboxylic acids;
- Functional Group: It has the carbonyl linked to a hydroxyl group (carboxyl group):
O
//
─C
oh
- Nomenclature: Acid + prefix + infix + oic.
- Examples: Methanic acid (formic acid) and ethanoic acid (acetic acid that forms vinegar).
7- Organic Function: esters;
- Functional Group: Derived from carboxylic acids, in which the carboxyl hydrogen (-COOH) is replaced by some organic group:
O
//
─C
O ─C
- Nomenclature: Prefix + infix + o + act / of / root name
- Examples: Pentyl ethanoate (banana flavor), ethyl butanoate (strawberry flavor) and isopentyl ethanoate (pear flavor).
8- Organic Function: ethers;
- Functional Group: It has the oxygen between two carbons: C ─ O ─ C;
- Nomenclature: minor group + oxy + major radical hydrocarbon;
- Examples: methoxyethane and ethoxyethane.
9- Organic Function: Amines;
- Functional Group: Derives from the replacement of one or more hydrogens of the ammonia group by carbon chains:
│ │
─ NH2 or ─ NH or ─ N ─
- Nomenclature: Prefix + infix + amine
- Examples: Methylamine, ethylamine and trimethylamine.
10- Organic Function: amides;
- Functional Group: It is theoretically derived from ammonia by replacing one of its hydrogens with an acyl group:
O
//
─C
NH2
- Nomenclature: Prefix + infix + amide.
- Examples: methanamide and ethanamide.
11- Organic Function: Nitrocompounds;
- Functional Group: It has the nitro group (NO2) linked to a carbon chain:
O
│ │
─ C ─ N ═ O
│
- Nomenclature: nitro + prefix + infix + o;
- Examples: nitromethane, nitroethane, 1-nitropropane and 2-methyl-1,3,5-trinitrobenzene (TNT).

TNT is an explosive that is a nitro compound.
12- Organic Function: Organic Halides;
- Functional Group: It has one or more halogens linked to a carbon chain:
X
│
─ C ─
X = F, Cl, Br or I.
- Nomenclature: amount of halogens + halogen name + hydrocarbon name;
- Examples: 2-bromopropane, chlorobenzene and 1,3-difluorobutane.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Identification of Organic Functions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/identificacao-das-funcoes-organicas.htm. Accessed on July 27, 2021.
Chemistry

Alkanes, hydrocarbons, methane, saturated chain, aliphatic, paraffins, fuels, gasoline, mineral wax, petroleum, oil shale, natural gas, petrochemical industry.
Chemistry

Alkenes, gas, ethene, ethylene, plastics, synthetic rubber, dyes, synthetic fabrics, explosives, petroleum cracking, polyethylene, olefiant gas, olefins, hydrocarbons, chain acyclic carbon dioxide.
Alkynes, ethic hydrocarbons, acetylenic hydrocarbons, acyclic carbon chain, carbon chain homogeneous, unsaturated carbon chain, triple bond, PVC, PVA, acetylene, synthetic rubbers, plastics, yarn textiles.
Chemistry

Aldehydes, carbonyl compounds, carbonyl group, Main aldehydes, Ethanal, raw material in the pesticide and drug industry, Metanal, formaldehyde, plastic and resin industry.
Amines, classification of amines, amine properties, primary amine, nitrogenous organic compounds, alkyl radicals, dimethylamine, ethylamine, trimethylamine, compounds extracted from vegetables, putrescine, cadaverine, organic bases, syntheses organic
Chemistry

Ketones, organic substances, carbonyl functional group, obtaining enamel solvent, propanone, ketone bodies in the bloodstream, extraction of oils and fats from plant seeds, solvents Organic.