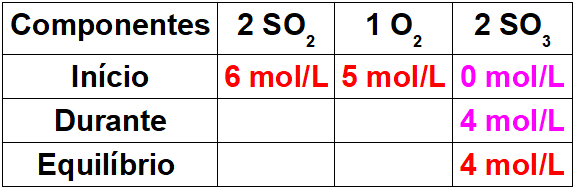
Before talking about the nomenclature of a quaternary ammonium salt properly, it is important to remember what this organic compound is. The quaternary ammonium salt originates from the replacement of hydrogens present in the ammonium cation (NH4+) per radicals organic, as shown below:
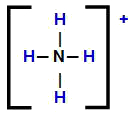
Ammonium cation structural formula
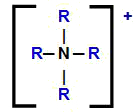
Structural formula of the ammonium cation with its hydrogens replaced by organic radicals
In addition to replacing hydrogens, every quaternary ammonium salt has any anion that accompanies the ammonium-derived cation. Thus, we can represent the general structural formula of this compound as follows:
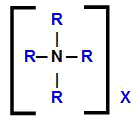
General structural formula of a quaternary ammonium salt
Thus, it is evident that, in the formula of a quaternary ammonium salt, we have the presence of four organic radicals, the same or different, and any anion (X). To carry out the nomenclature of these compounds, we have to take this fact into account.
See how the nomenclature of a quaternary ammonium salt according to IUPAC (International Union of Pure and Applied Chemistry):
Anion name + de + name of the radicals in alphabetical order + ammonium
Now follow some examples of application of the rule of nomenclature of quaternary ammonium salts:
Benzyl-octadecyl-dimethylammonium iodide
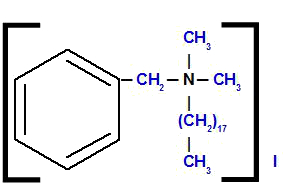
Analyzing the structure, we observe that the anion present in the compound is the I, which is called iodide. To the left of the structure, we have the radical benzyl (junction of benzene with the CH group2). On top and on the right, we have two radicals methyl (CH3), which will be called 'dimethyl'. At the bottom, we have the radical octadecyl, which has eighteen carbon atoms in its chain.
Do not stop now... There's more after the advertising ;)
Thus, the name starts with iodide, followed by the preposition of, plus the name benzyl, followed by octadecyl (because of the alphabetical order) and, finally, dimethyl with ammonium.
Observation: The terms oct and di do not participate in the alphabetical sequential organization of the compound name.
tetraethylammonium chloride
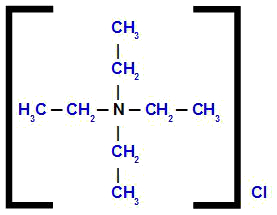
Analyzing the structure, we observe that the anion present in the compound is the Cl, which is called chloride. The four organic radicals present in the structure are ethyl (CH3-CH2-); therefore, they receive the name tetraethyl.
Thus, the name starts with chloride, followed by the preposition of, plus the name tetraethyl, followed by the term ammonium.
Benzyl-tridecyl-dimethylammonium bromide

Analyzing the structure, we observe that the anion present in the compound is the br, which is called bromide. To the left of the structure, we have the radical benzyl (junction of benzene with the CH group2). On top and on the right, we have two radicals methyl (CH3), which will be called 'dimethyl'. At the bottom, we have the radical tridecil, as there are thirteen carbon atoms in its chain.
Thus, the name starts with iodide, followed by the preposition of, plus the name benzyl, followed by tridecyl (because of the alphabetical order) and, finally, dimethyl with ammonium.
Diphenyl-dimethylammonium sulfate
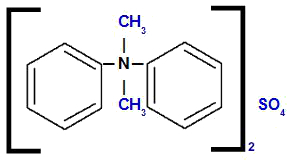
Analyzing the structure, we observe that the anion present in the compound is the ONLY4, which is called sulfate. On the left and right of the structure, we have the phenyl radical (benzene), which will be called 'diphenyl'. At the top and bottom we have methyl radicals (CH3), which will be called 'dimethyl'.
Thus, the name starts with sulfate, followed by the preposition of, plus the name diphenyl, followed by dimethyl (because of the alphabetical order) and, finally, ammonium.
By Me. Diogo Lopes Dias