We know that the air exerts a pressure on us and all other bodies on the Earth's surface that we know as atmospheric pressure. The atmospheric pressure value was determined using a device called a barometer. This instrument was used by physicist Evangelista Torricelli, for this reason we know this device Torricelli barometer. So let's see what the Torricelli barometer is.
we name Torricelli barometer the apparatus consisting of a long tube (1 meter) of glass and a vat, also made of glass, containing mercury. The glass tube is completely filled with mercury, the open surface of the glass tube being blocked by the thumb. Then the tube is inverted in the tub and the finger is removed. The mercury level drops to stabilize at a height h above the surface of the mercury in the vat. In the region of the tube, above the mercury column, there is the barometric chamber, a region of very low pressure.
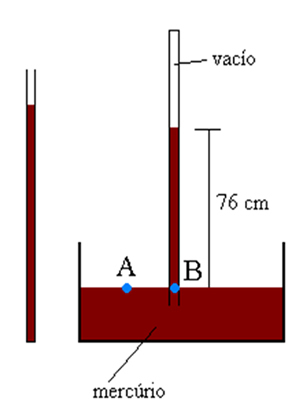
How can we explain why mercury stabilizes inside the glass tube at a certain point H? Evangelista Torricelli explained that atmospheric air exerts pressure on the entire free surface of the mercury contained in the vat, this pressure being capable of sustaining the mercury column inside the pipe.
Do not stop now... There's more after the advertising ;)
According to Stevin's theorem, points A and B, located in the same liquid and in the same horizontal plane, are under the same pressure. Stevin defined that at point A the pressure exerted corresponds to atmospheric pressure, while at point B it is the pressure of the column of mercury of height h that acts. Therefore, it was concluded that:
Patm=μ.g.h
Torricelli noted that at a location where the acceleration of gravity was 9.8 m/s2 and that the temperature was 15ºC, at sea level, the height H it was equal to 76 cm. Torricelli concluded that this pressure was exactly normal atmospheric pressure.
To obtain the atmospheric pressure value in its corresponding Pascal, just do:
Being,
m.g.h = 13.6 .103 kg/m3
g = 9.8 m/s2 and h = 76 cm = 0.76 m
We have the pressure of
1 atm=76 cmHg = 1.013.105 Pan
By Domitiano Marques
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
SILVA, Domitiano Correa Marques da. "The Torricelli Barometer"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/o-barometro-torricelli.htm. Accessed on June 27, 2021.