The burning of fuels (such as wood, natural gas, oil or coal) has been used for hundreds of years by humanity to produce thermal energy. We burn butane gas for cooking, gasoline to power cars, coal to produce electricity. In all of these processes, combustion is used to generate heat from an exothermic chemical reaction that oxidizes the fuel.
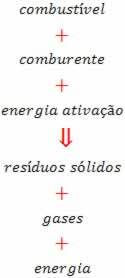
This reaction can be seen in the figure below:
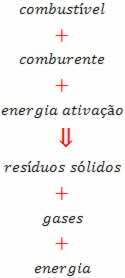
The fuel can be gas, gasoline, wood or coal. The oxidizer can be the oxygen gas in the air; and activation energy can be a spark. The gases generated are the H2O, CO2, CO, NO2, ONLY2 and others.
Solid waste is ash and heavy metal components. Only the presence of fuel and oxidizer does not guarantee the start of the combustion process. It is necessary to give a minimum of thermal energy to start the reaction. Activation energy is the energy needed to start the combustion process. Once the reaction starts, it ends when the fuel runs out.
Some combustion results are:
- the production of a lot of energy in the form of heat, which is used in different ways, such as simple space heating, the generation of thermoelectric energy and the operation of cars.
Do not stop now... There's more after the advertising ;)
- fuel consumption. When we use fuels from non-renewable sources, such as oil, or its derivatives, we are burning a substance that nature took hundreds of thousands of years to manufacture, and whose reserves are finite. Therefore, over time, the tendency is for these reserves to run out or it will be very difficult to extract them cheaply.
- another result of burning organic fuels is the increase in air pollution. Gases like SO, SO2, NINTH2 and NO3 they combine with water vapor present in the atmosphere, resulting in sulfuric acid and nitric acid, which return to the Earth's surface in the form of acid rain. Other gases such as CO and CO2, released into the atmosphere, contribute to the greenhouse effect, which can cause an increase in the planet's average temperature.
The thermodynamic study allows us to use, in a rational way, the natural fuels that we can extract from nature. In addition to optimizing the burning process, we can reduce atmospheric pollution, reuse solid waste as much as possible and build increasingly more efficient machines.
Currently, a lot of effort is being made to use renewable energy sources, such as solar energy and alternative fuels, such as alcohol and hydrogen.
By Domitiano Marques
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
SILVA, Domitiano Correa Marques da. "Combustion"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/combustao.htm. Accessed on June 27, 2021.