Organic compounds can be represented in a variety of ways, such as a flat structural formula, a simplified or condensed structural formula, or a dash formula. However, the simplest representation is via the molecular formula.

Thus, let's see how to determine the molecular formula of organic compounds, based on the other formulas mentioned above.
1. Through the Flat Structural Formula:this formula shows the arrangement or arrangement of atoms within the molecule. For example, below is the flat structural formula of one of the hydrocarbons present in gasoline.

Note that, in this formula, all atoms and all existing bonds between them are shown. Now, to determine the molecular formula of this compound, just count the number of atoms of each element and place an index on the lower right side of the element in question.
An important aspect to be highlighted is that we always start the molecular formula of organic compounds from the element carbon, as it is the main constituent of these substances. See the example:
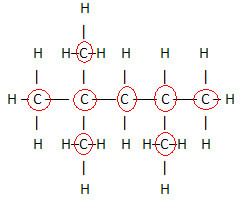
Since there are 8 carbons, we start writing the molecular formula like this: Ç8
To complete this formula, we count the amount of hydrogens:
So your molecular formula é Ç8H18.
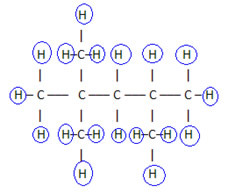
2. Through the simplified or condensed structural formula: in this type of formula, the amount of hydrogens is abbreviated. For example, look at the same formula for the molecule found in gasoline, now in a condensed form:
Do not stop now... There's more after the advertising ;)
This way it is even easier to count the amount of hydrogens, just add the indices (3 +3+ 3 +2 +1 +3 +3 = 18).
But now let's look at the condensed structural formula of linoleic acid, which exists in vegetables such as cotton, soybeans, sunflowers, etc. and which is used in paints and varnishes:
H3C─CH2─CH2─CH2─CH2CH═CH─CH2CH═CH─CH2─CH2─CH2─CH2─CH2─CH2─CH2─COOH
Counting the amount of carbons, hydrogens and oxygens, we have the following molecular formula of linoleic acid: Ç18H32O2.
3. Through the stroke formula: this formula further simplifies how to represent organic compounds, as it omits the groups C, CH, CH2 and CH3.
An example is the linoleic molecule, see how it looks:

Let's count the amount of carbons first, remembering that, in this formula, each bond between carbons is represented by the dash. Thus, the tips, as well as the two points of inflection, correspond to carbon atoms.
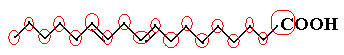
So we have: Ç18
Now, to count the amount of hydrogens, we have to remember that the bonds between carbons and hydrogens are implied, as carbon is known to make four bonds; thus, the amount of bonds that are missing is the amount of hydrogen bonded to that element.
See the explanations below:
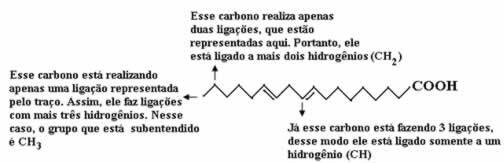
Thus, the amount of hydrogens will be: 32.

The amount of oxygen is quite simple to count, as there are only two. Since the molecular formula é: Ç18H32O2.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Molecular Formulas of Organic Compounds"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/formulas-moleculares-compostos-organicos.htm. Accessed on June 28, 2021.
Carbon spatial formula, Lewis Electronic Formula, plane structure, electronic pairs, bond covalent, valence layer, evolution of the atomic model, molecular formula, structural formula, formulas three-dimensional.
Chemical formulas, flat structural formula, Couper structural formula, triple bond, gas nitrogen, electronic formula, Lewis formula, molecular formula, single bond, double bond, gas carbonic.



