Catalysts are substances capable of accelerating a reaction without being altered, that is, they are not consumed during the reaction.
To understand how catalysts work, we need to remember what was explained in the text "Activation energy”. As shown there, for a chemical reaction to start, it is necessary that the reactants have or receive a certain amount of minimum energy, which is called activation energy.
With this minimum energy, the reactants are able to reach the complex activated, which is an intermediate state (transition state) that is formed between reactants and products, in which structure exist the weakened previous links and the formation of new links (present in the products).
For example, consider the generic reaction below:
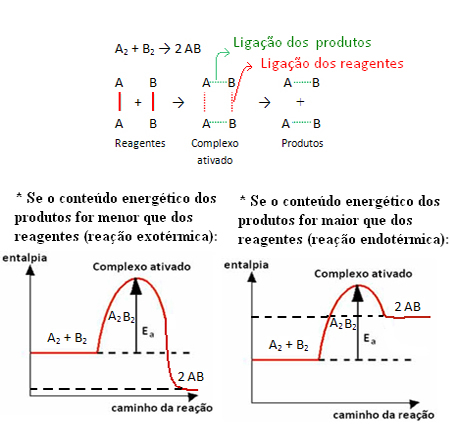
Note that the activation energy needed to reach the activated complex becomes a kind of hurdle that needs to be overcome for the reaction to take place. This means that the greater the activation energy of a reaction, the greater the obstacle to be overcome and the slower the reaction speed.
The opposite is also true, if the activation energy is lower, the reaction will be faster. That's exactly what the catalysts do they they create an alternative path, which requires less activation energy, making the reaction proceed faster.
Do not stop now... There's more after the advertising ;)

In order to lower the activation energy, the catalyst acts by changing the reaction mechanism, by combining with the reagents in a system that can be monophasic (homogeneous catalysis) or polyphasic (heterogeneous catalysis).
More details about these types of catalysis can be seen in the texts below:
- homogeneous catalysis
- heterogeneous catalysis
But, generally speaking, we can say that this The combination of the reactant and the catalyst forms an intermediate compound which then transforms, giving rise to the product and the catalyst. Note how this can be represented:
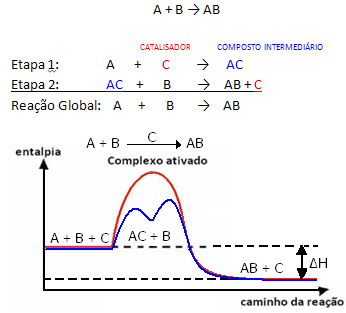
See that the catalyst is regenerated at the end of the reaction, not being consumed by it.
An important fact is that the catalyst accelerates both the forward and reverse reactions, that means it decreases the activation energy of both.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "How do catalyst substances act?"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/como-atuam-as-substancias-catalisadoras.htm. Accessed on June 27, 2021.