Geometric isomerism or cis-trans it occurs in aliphatic compounds and in cyclic compounds. In the text "Igeometrical summation or cis-trans” you've seen about this kind of stereoisomerism in open-chain compounds, now let's see when it occurs in cyclic compounds.
Geometric spatial isomerism or cis-trans occurs in closed-chain compounds that have two or more different groups attached to at least two carbon atoms in the chain. But, these different ligands on one carbon atom must be the same as the ligands on the other carbon atom.
For example, consider the case of bromocyclopentane:

Do not stop now... There's more after the advertising ;)
this compound no presents geometric isomerism cis-trans, as it has only one different ligand, bromine. Now look at the compound below:
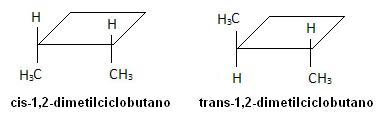
Two carbons in this structure have different ligands and the same as the other carbon atom. In the first case, the same ligands are on the same side of the plane, so this is a stereoisomer. cis. In the second molecule, the same ligands are on opposite sides of the plane, being called trans.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Cis-trans geometric isomers in cyclic compounds"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/isomeria-geometrica-cis-trans-compostos-ciclicos.htm. Accessed on June 28, 2021.


