Plants carry out photosynthesis reactions, in which water, carbon dioxide and the sun's energy retained by chlorophyll produce oxygen and glucose, which is a carbohydrate classified as a monosaccharide:
6 CO2(g) + 6 H2O(ℓ) + sunlight→ 1 Ç6H12O6(aq)+ 6 O2(g)
Glucose molecules combine to form polysaccharides, which are naturally occurring condensation polymers. When this union occurs by β-glucose units, cellulose is formed:

Each cellulose molecule is made up of 10,000 or more β-glucose units
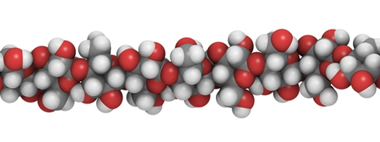
Cellulose constitutes the cell wall of all plants and is an external reinforcement of plant cells. The β bond makes this polysaccharide have a very rigid structure. Unlike starch and glycogen, which are polysaccharides formed by the union of α-glucose molecules.
For this reason, humans cannot digest ingested cellulose. Some animals, such as ruminants, which include deer and ox, are able to digest the cellulose because have bacteria in their digestive tracts that produce enzymes capable of metabolizing this polymer. Termites are also capable of this because they have protozoa that produce the enzymes that do this job.
Do not stop now... There's more after the advertising ;)
However, even not being able to metabolize cellulose, the ingestion of these fibers, through green leafy salads, for example, is important for the daily diet of human beings, because cellulose serves as a means for the development of beneficial bacteria and also helps in the proper functioning of the intestine and excretion of feces, stimulating the production of saliva and juice. gastric.
The plant that has the most cellulose is cotton (Gossypium sp.), with 98% in mass, being used by the industry in the production of fabrics and personal care and aesthetic materials, such as cotton rolls and cotton swabs. Wood is 50% cellulose, which is mainly used in paper production.

By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Cellulose"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/celulose.htm. Accessed on June 27, 2021.
Chemistry

Wood, woody fabric from trees, cellulose pulp, rayon, tar, tannin, cellulose acetate, leather tanning, cellulose trinitrate, cotton powder, manufacturing of explosives.