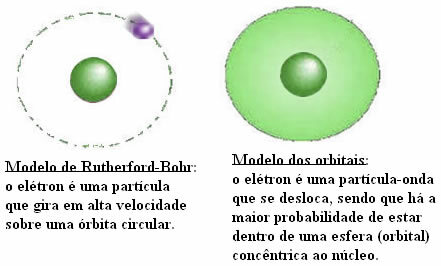
Transition elements are also known as Transition Metals. The name is appropriate, as the properties of these elements are intermediate between the elements. metallics, which are on the left of the Table (Alkaline and Alkaline-earth metals), and the elements not metallic.
The members of the Periodic Table that are part of this denomination are classified into Internal or External Transition Elements, as in the table below:

Note that transition metals refer to elements belonging to groups 3 to 12 (in green color), located in the center of the Table.
External transition elements - they are presented on three levels:
First transition series: titanium, vanadium, chromium, manganese, iron, cobalt, nickel and copper.
Second transition series: zirconium, niobium, molybdenum, technetium, ruthenium, rhodium, palladium and silver.
Third transition series: Hafnium, Tantalium, Tungsten, Rhenium, Osmium, Iridium, Platinum and Gold.
These elements generally have the 3d, 4d and 5d valence orbitals (more energetic sublevels).
Internal transition elements (or just Transition elements):
Lanthanides: elements ranging from atomic number 57 to 71.
Actinides: elements ranging from atomic number 89 to 103.
Lanthanides and actinides have 4f and 5f valence orbitals.
The transition elements, both internal and external, are metals and have high thermal and electrical conductivity.
By Líria Alves
Graduated in Chemistry
Do not stop now... There's more after the advertising ;)
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Transition Elements in the Periodic Table"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/elementos-transicao-na-tabela-periodica.htm. Accessed on June 28, 2021.