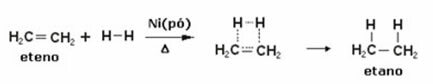
In order for you to understand which compounds constitute the class of terpenes, let's first analyze what are the alkadienes or simply, dienes.
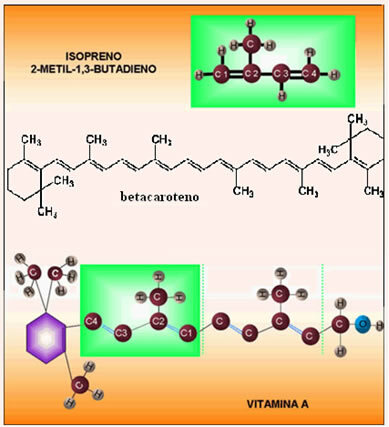
Alkadienes are unsaturated hydrocarbons with two double bonds. Among these organic compounds we have the isoprenes, which have the following chemical structure:
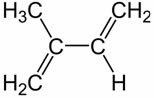
The official nomenclature for this compound is 2-methyl-but-1,3-diene. It is important to know this structure presented because the terpenes are a class of organic compounds that are made up of “isoprene units”.
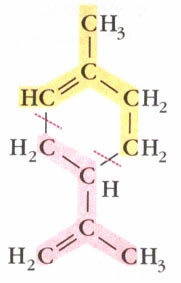
For example, a terpene named limonene is an oil found in the peel of fruits such as lemon and orange and its structure corresponds to two isoprene units linked together, forming a ring. The structure of limonene is shown below and the two isoprene units that form it are divided into different colors for better visualization:
Other terpenes are found in fruit peels, seeds, flowers, leaves, roots, vegetables, woods, etc. Those oils that are extracted usually have a very pleasant smell and are even used to produce perfume fragrances or as flavoring agents.
O myrcene is another isoprene found in bay leaf oils and verbena flower oils. Like limonene, myrcene has only two isoprene units, but with an open chain:
CH3 ─ C ═ CH ─CH2CH2─ C ─ CH═ CH2
│║
CH3 CH2
Do not stop now... There's more after the advertising ;)
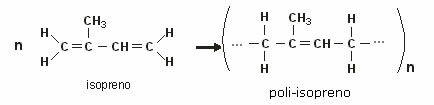
Another example of an isoprene polymer is the main component of latex (natural rubber), found in the sap of various trees. The figure below shows the rubber formation reaction:
The best known isoprene is beta carotene, which is responsible for the orange color of the carrot. Its structural formula is formed by 40 carbons and 11 alternating double bonds. Its electrons move freely because of this large amount of conjugated double bonds and therefore vibrate with the same energy in the wavelength of light with a blue-green color. Therefore, the electrons from the double bonds of beta-carotene absorb this wavelength of light and reflect a resultant light in the wavelength range in the orange color.
The consumption of foods with beta-carotene is important, as its molecule is transformed in our body into vitamin A, which is another terpene. Vitamin A helps with vision, and its deficiency can cause night blindness. In addition, vitamin A is an antioxidant food, that is, its molecules combine with free radicals in our body, making them harmless compounds. Otherwise, free radicals can damage healthy cells in our bodies and cause premature aging.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Terpenes"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/terpenos.htm. Accessed on June 28, 2021.