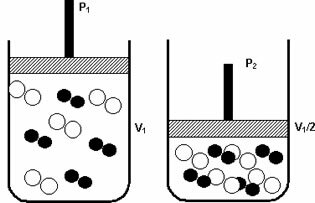
question 1
Given the aqueous solutions of the same molar concentration, which have the following solutes:
I. Ç6H12O6
II. Ç6H8O6
III. KNO3
IV. Ç63H88Con14O14P
V. NaCl
The solutions that have the lowest vapor pressure are, respectively:
a) I and II.
b) III and IV.
c) III and V.
d) II and V.
e) I and IV.
question 2
See the following aqueous solutions and their respective concentrations:
I- 0.50 mol/L of C6H12O6
II- 0.15 mol/L of C6H12O6
III- 0.25 mol/L of C6H12O6
IV- 0.35 mol/L of C6H12O6
IV- 0.45 mol/L of C6H12O6
Check the alternative that presents the solution with the highest and lowest vapor pressure respectively:
a) I and II
b) II and III
c) II and IV
d) II and I
e) V and III
More questionsWatch our video class to learn about the history of Islam. Also check our channel for further information about the Middle Ages.
Considering the dynamism of the Portuguese language, one cannot deny the importance of studying linguistic varieties, a theme recurrently raised in Enem. In this video class, we will analyze, in practice, how questions about this content are presented. Check out!