There is a branch of science that studies the speed of chemical reactions and the factors that influence it, it is called Chemical Kinetics. Chemical reactions can be defined as a set of phenomena in which two or more substances react with each other, giving rise to different compounds. A chemical equation is a graphical representation of a chemical reaction, where reactants appear in the first member, and products in the second.
A + B  C + D
C + D
Reagents Products
The knowledge and study of reactions, in addition to being very important in industrial terms, are also related to our daily lives.
The speed of a reaction is how quickly reactants are consumed or how quickly products are formed. The burning of a candle and the formation of rust are examples of slow reactions. In dynamite, the decomposition of nitroglycerin is a rapid reaction.
The speeds of chemical reactions are determined through empirical laws, called laws of speed, deduced from the effect of the concentration of reactants and products on the speed of reaction.
Chemical reactions occur at different speeds and these can be changed, because in addition to concentration of reactants and products, reaction rates also depend on other factors like:
Reagent concentration: the higher the concentration of the reactants, the faster the reaction. For a reaction to take place between two or more substances it is necessary that the molecules collide, so that there is a break in the bonds with the consequent formation of new ones. The number of collisions will depend on the concentrations of A and B. See the picture:
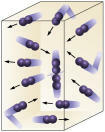
Molecules collide more often if
we increase the number of reacting molecules.
Do not stop now... There's more after the advertising ;)
It is easy to see that due to a higher concentration there will be an increase in collisions between molecules.
contact surface: an increase in the contact surface increases the reaction speed. An example is when we dissolve a crushed sonrisal tablet and it dissolves faster than if it were whole, this happens because we increase the contact surface that reacts with the Water.
Pressure: when you increase the pressure of a gaseous system, the reaction speed increases.
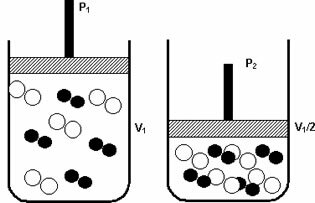
An increase in pressure from P1 to P 2 reduced the volume from V1 to V1/2, accelerating the reaction due to the approach of the molecules.
The figure above exemplifies, as with the decrease in volume in the second container, there will be an increase in the pressure intensifying the collisions of the molecules and as a consequence an increase in the speed of the reaction.
Temperature: when the temperature of a system is increased, there is also an increase in the reaction rate. Increasing the temperature means increasing the kinetic energy of molecules. In our day to day we can observe this factor when we are cooking and we increase the stove flame so that the food reaches the degree of cooking faster.
Catalysts: Catalysts are substances that accelerate the mechanism without undergoing permanent alteration, that is, during the reaction they are not consumed. Catalysts allow the reaction to take an alternative path, which requires less activation energy, causing the reaction to proceed more quickly. It is important to remember that a catalyst speeds up the reaction, but does not increase the yield, that is, it produces the same amount of product, but in a shorter period of time.
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Chemical Kinetics"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/cinetica-quimica.htm. Accessed on June 27, 2021.