Consider the generic reversible reaction below, where the lower case letters correspond to the balanced reaction coefficients and the upper case letters are the reactants and the products are all gaseous:

Considering each of the reaction directions separately, their development rates (Td) are given by:
*Direct reaction: aA + bB → cC + dD
Alldirect = Kdirect. [THE]The. [B]B
*Reverse reaction: cC + dD → aA + bB
Allinverse = Kreverse. [Ç]ç. [D]d
The chemical equilibrium constant in terms of concentration in quantity of matter (Kç) and in terms of partial pressure (KP) will be given by dividing Kdirect by Kreverse.
So we have:
Kdirect. [THE]The. [B]B = 1 → Kdirect__ = __[Ç]ç. [D]d___
Kreverse. [Ç]ç. [D]d Kreverse [THE]The. [B]B
Being, Kç =_Kdirect_
Kreverse
So we have:
Kç =__[Ç]ç. [D]d___ or KP =__(Praça)ç. (pD)d___ |
Where p is the partial pressure of each substance at equilibrium.
In this way, each concentration is raised to an exponent corresponding to the coefficient of the respective substance in the reaction, and Kç does not have unit*.
In addition, a very important aspect to be highlighted is that in this expressionneither solid components nor pure liquids should be represented., as only matters that can suffer variation participate in this expression. The concentration in amount of matter of a substance in the solid state is constant and thus is already included in the value of K itself.ç. The same goes for pure liquids like water. In short, only substances in the gaseous state and in aqueous solution participate in the expression.
Do not stop now... There's more after the advertising ;)
Note the examples below:
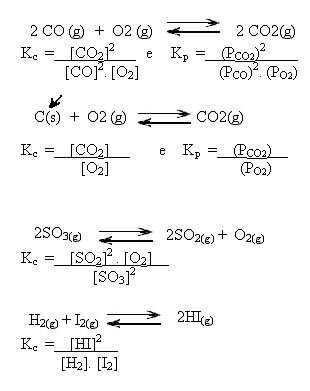
K valuesç can show us if the concentration of reactants and products are the same or if one is greater than the other:
- if Kç or KP is equal to one (Kç = 1), this means that the concentration of reactants and products is equal;
- if the value of Kç or KP be tall, this means that the products are in greater concentration, because in the expression of Kç the products are in the numerator;
- if the value of Kç or KP is low, this means that the reagents are in higher concentration, because in the expression of Kç the reagents are in the denominator.
*Kç and KP they are dimensionless numbers, that is, pure numbers, with no unit related to any magnitude or relation between magnitudes.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Equilibrium Constants Kc and Kp"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/constantes-equilibrio-kc-kp.htm. Accessed on June 28, 2021.


