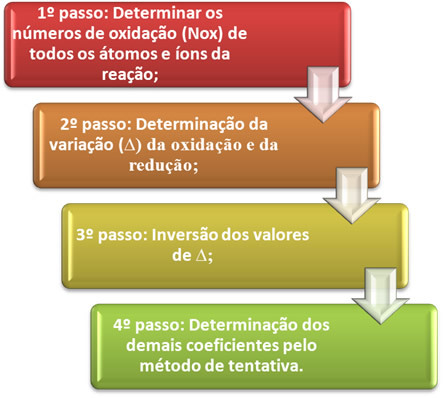
O balancing an oxidation-reduction equation it is based on the equality of the number of electrons given away with the number of electrons received. A simple method of performing this balancing is given by the following steps:
Let's see in practice how to apply these steps, through the following example:
Reaction between an aqueous solution of potassium permanganate and hydrochloric acid:
kmnO4 + HCl → KCl + MnCl2 + Cl2 + H2O
*1st step:Determine oxidation numbers:
This step is important because we usually cannot quickly visualize which species undergo oxidation and reduction.
+1 +7 -2 +1 -1 +1 -1 +2 -1 0 +1 -2
kmnO4 + HCl → KCl + MnCl2 + Cl2 + H2O
*2nd step:Determination of oxidation and reduction variation:

Note that manganese (Mn) is reduced and chlorine (Cl) is oxidized.
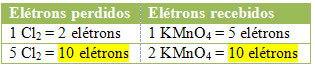
MnCl2 = ∆Nox = 5
Cl2 = ∆Nox = 2
In the case of chlorine, we can note that HCl gave rise to 3 compounds (KCl, MnCl2, and Cl2), but what interests us is the Cl2, because it is your Nox that has suffered variation. Each chlorine that forms Cl
2 lose 1 electron; how it takes 2 chlorines to form each Cl2, then two electrons are lost.3rd step:Inversion of ∆ values:
In this step, the values of ∆ are exchanged between the mentioned species, becoming their coefficients:
MnCl2 = ∆Nox = 5 → 5 will be the coefficient of Cl2
Cl2 = ∆Nox = 2→ 2 will be the coefficient of MnCl2
kmnO4 + HCl → KCl + 2 MnCl2 + 5 Cl2 + H2O
At this point it is already possible to know two coefficients of the equation.
Observation: normally, in most reactions, this reversal of values is performed on the 1st member. But, as a general rule, this should be done in the member that has the greatest number of atoms that undergo redox. If this criterion cannot be met, we invert the values for the member with the highest number of chemical species. This is what was done here, as the 2nd member has more substances.
Do not stop now... There's more after the advertising ;)
4th step: Trial balancing:
kmnO4 + HCl → KCl + 2 MnCl2 + 5 Cl2 + H2O
- Since in the second member there are two manganese atoms, as shown by the coefficient, in the first there must also be. So we have:
2 kmnO4 + HCl → KCl + 2 MnCl2 + 5 Cl2 + H2O
- Thus, the amount of potassium (K) in the 1st member was 2, which will be the same coefficient for this atom in the second member:
2 kmnO4 + HCl → 2 KCl + 2 MnCl2 + 5 Cl2 + H2O
- The amount of chlorine (Cl) in the 2nd member is 16 in total, so the HCl coefficient of the 1st member will be:
2 kmnO4 + 16 HCl → 2 KCl + 2 MnCl2 + 5 Cl2 + H2O
- The number of hydrogens in the 1st member is 16, hence the coefficient of water (H2O) of the 2nd member will be equal to 8, since the multiplication of the hydrogen index (2) by 8 is equal to 16:
2 kmnO4 + 16 HCl → 2 KCl + 2 MnCl2 + 5 Cl2 + 8 H2O
- To check if the equation is correctly balanced we can see two criteria:
1st) Check if the amount of each atom in the two members is equal:
2 kmnO4 + 16 HCl →2 KCl + 2 MnCl2 + 5 Cl2 + 8 H2O
K =2K =2
Mn = 2 Mn = 2
Cl = 16 Cl = 16
H = 16 H = 16
O = 8 O = 8
2nd) See if the total number of lost electrons is equal to the total number of electrons received:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Oxidation-reduction balancing"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/balanceamento-por-oxirreducao.htm. Accessed on June 28, 2021.