The Hybridization Theory emerged as a complement to the Octet Theory, managing to explain the structure of a larger number of molecules, including molecules formed by boron (B).
This element has five ground state electrons, with the following electronic configuration:
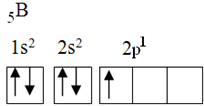
By the octet theory, boron could only make one covalent bond, as it has only one incomplete atomic orbital. However, experimentally, it is noted that this element forms compounds in which it performs more than one bond.
An example is boron trifluoride (BF3). As shown below, boron makes three covalent bonds with fluorine, sharing three pairs of electrons and having six electrons in its last shell (valence layer), ie, an exception to the rule of octet.
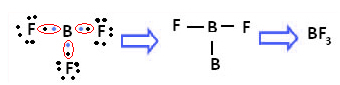
This is explained by the hybridization phenomenon that occurs with boron. It turns out that an electron from sublevel 2s absorbs energy and goes into the excited state, in which it jumps into an empty orbital of sublevel 2p. In this way, three incomplete orbitals are formed, which can now make three covalent bonds:
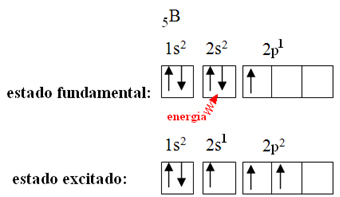
However, the bonds formed in boron trifluoride are all the same, but if we look above, there are two bonds different, since one of them would be made through an s orbital and the other two through an orbital of the type. type p. This is where the hybridization takes place, that is, the incomplete orbitals merge, giving rise to three hybrid orbitals or hybridized, which are identical and different from the originals:
Do not stop now... There's more after the advertising ;)
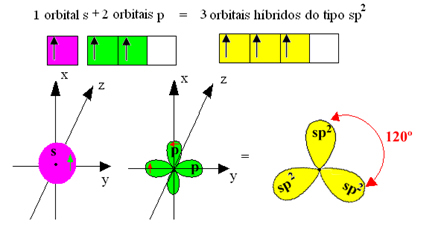
Since these hybrid orbitals consist of one s orbital and two p orbitals, this hybridization is called sp² hybridization.
The fluorine that binds to boron has nine electrons. Its electronic distribution and orbitals are shown below:
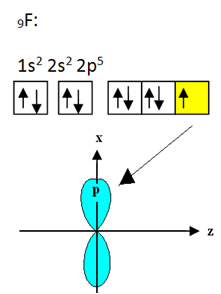
Note that each fluorine atom can make only one covalent bond and that the orbital that makes this bond is of type p. So, observe below how the orbitals are formed when making the connections that form the BF3 and see how the links are identical, like σp-sp2:
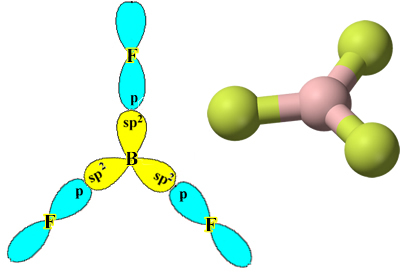
This also happens with other elements, see, for example, the text “Beryllium Hybridization”.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Boron hybridization"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/hibridizacao-boro.htm. Accessed on June 28, 2021.


