O kerosene is a known liquid derived from Petroleum, mainly because it is the fuel used in airplanes. From 1800, the kerosene began to be used in lamps. Nowadays, there are several ways to light a place, such as a camp, but kerosene is still used for this purpose.

Lamp model used for lighting
The use of this liquid on a daily basis is often reduced to dissolving a component apolate, even more so taking into account its unpleasant odor.
In this text, our objective is to address the particularities and, mainly, about the kerosene chemistry.
method of obtaining
The method of obtaining the kerosene it is physical, with the use of oil, in the process of separation of mixtures called fractional distillation. As oil is a homogeneous mixture formed by several liquids, this is a possible way to separate them.
O kerosene It is obtained when, in oil distillation, thermometers measure temperatures around 150 °C and 300 °C. This temperature is intermediate in relation to obtaining gasoline and diesel oil liquids.

Schematic drawing of the oil distillation tower
The chemistry of kerosene
Because it is one of the components of oil, and this is a mixture of Hydrocarbons, O kerosene it also has this composition, however, it is formed by hydrocarbons that have 9 to 13 carbons, the majority aromatic, derived from naphthalene and phenanthrene.
Do not stop now... There's more after the advertising ;)

Structural formula of naphthalene
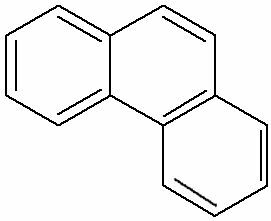
Structural formula of phenanthrene
Main characteristics of kerosene
After being obtained in fractional distillation, the kerosene may undergo some physicochemical treatments, according to its purpose:
→ Post-distillation (basic kerosene): It has basic characteristics, which are verified in any type of kerosene.
Insoluble in water;
Characteristic and unpleasant odor;
Clear liquid;
Less dense than water;
- Very toxic to living beings.
→ For aviation:
Maximum combustion efficiency;
High calorific value;
Minimal tendency to waste formation;
Absence of corrosivity;
low freezing point;
- Low vapor pressure.
→ For lighting or industrial:
Clear liquid;
Low sulfur content;
Flash point must be at least 40°C;
Produces odor and smoke free burning.
General uses of kerosene
Fuel for jet aircraft turbines;
Industrial solvent;
In the manufacture of substances (explosives, for example);
Extraction agent for substances present in mixtures;
Used in coatings (such as paints and adhesives);
Used as a solvent in road and construction applications;
binding agent or release agent for metals;
Use in agrochemicals;
- Used as a functional fluid (whether in transfer fluids, insulating coolants in machinery, hydraulics, etc.).
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "The chemistry of kerosene"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/a-quimica-querosene.htm. Accessed on June 28, 2021.


