When the benzene ring already has a substituent, this radical will influence all other H substitutions on the ring. This substituent can be ortho and para-director or meta-director. But the questions arise:
| What causes the group attached to the benzene ring to influence the direction and reactivity of the substitution reaction? |
| What makes certain groups goal advisors (disabling) and others ortho-para (activating)? |
These two questions are answered by understanding the callings. electronic effects that these groups exercise in the ring. This effect occurs due to the electronegativity difference between the elements, as the substituent will polarize the bonds of the aromatic nucleus, alternately inducing a positive character to some ring carbons, while others remain with a negative character.
| A new substitution will occur in the carbon atoms that have character negative. |
Let's see how these electronic effects occur in the aromatic ring, keeping in mind the order of electronegativity of the elements: F > O > N > Cl > Br > S > C > I > H.
1st case: Radical activating or ortho-to-director:
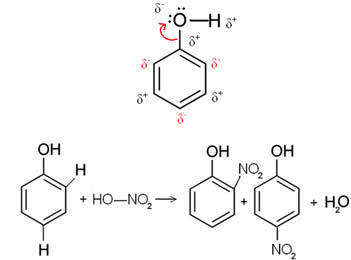
For example, in the case below the benzol (phenol) molecule, oxygen is the most electronegative element, so it attracts electrons to itself, causing the carbon to have a partially positive charge, which induces alternating ring polarization. The positions that are negative are exactly the ortho and para positions. That is why the -OH group is an activating radical or ortho-to-directors. This can be seen in the phenol nitration reaction below, giving rise to o-nitrophenol and p-nitrophenol as products:
Do not stop now... There's more after the advertising ;)
2nd case: deactivating radical or meta-leader:
Now consider the case of nitrobenzene:
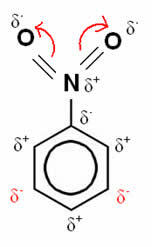
In this example, oxygen remains the most electronegative element, so it attracts the bonds made with nitrogen to itself, which is partially positively charged, inducing the carbon atom attached to it to become negatively polarized and so successively. Thus, the position that becomes negative and most susceptible to substitution is the position goal, being, therefore, a disabling.
See now in more detail this electronic effect, which is called resonant effect.
| resonant effect it is the attraction or repulsion of electrons on π (pi) bonds of double or triple bonds, when they resonate with the benzene ring itself. |
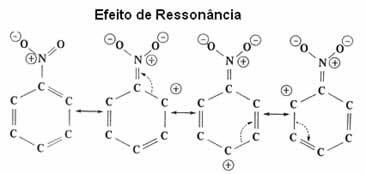
As seen, the NO2 group is deactivating the ring, as it is taking electrons from it and decreasing its electron density. Thus, the group that will attack and make the substitution (electrophile) is positive, so it will preferentially attack the meta position that has a negative charge.
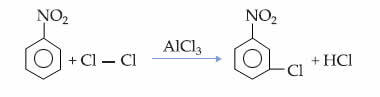
This fact can be seen in the monochlorination reaction of nitrobenzene, in which the substitution occurs only in the meta position:
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Electronic effects of meta and ortho-to-directors radicals"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/efeitos-eletronicos-radicais-meta-orto-para-dirigentes.htm. Accessed on June 28, 2021.