The text Addition Reactions showed that these types of organic reactions are named this way because a reactant is added to the organic molecule by breaking bonds between carbons. In this text the case of alkenes was shown, here we will already consider how this occurs with alkynes, or that is, with those hydrocarbons (formed only by carbon and hydrogen atoms) that have a bond triple.
The vulnerable point of alkynes is exactly the triple bond, where pi (π)-type bonds can be broken one (addition partial) or twice (total addition) and give rise, respectively, to new compounds with double (alkenes) or single bonds (alkanes).
Let's look at the cases of addition reactions in alkynes:
1. Addition of hydrogen or hydrogenation:
In this case, the H molecule2 is added to alkyne using a catalyst, which is usually powdered nickel (Ni), platinum (Pt) or palladium (Pd). Due to the need to use a catalyst, this reaction is also called catalytic hydrogenation and it occurs in stages: in the first stage you get an alkene, and in the second stage, which is slower, you get an alkane.
If the catalyst used is strong, such as nickel and platinum, the reaction directly produces alkane. Palladium mixed with BaSO4 it is a weak catalyst and produces alkene. It is also possible to use a partial catalyst inhibitor to only reach the alkene. This reaction also takes place under high pressures and temperatures.
Next, we have the addition of hydrogen to ethane, producing ethylene and then ethane:

2. Addition of halogens or halogenation:
An alkyne pi bond is broken and two halogen atoms are added to the molecule (elements from the 17A family of the Periodic Table, the most used being: Cl2 and Br2), forming a vicinal dihalide, meaning that two halogen atoms are bonded to neighboring carbon atoms. The reaction can continue, breaking the other pi bond and adding two more halogen atoms to the molecule.
In the example below, we have this type of addition to the bribe:
ClCl ClCl
││ ││
H ─C ≡ C CH3 + Cl2 → H ─ C ═ C CH3 + Cl2 → H ─ C ─ C CH3
││
ClCl
ALCINO DI-HALETTE TETRAHALETTE
3. Addition of hydrogen halides (halogenhydrides or hydrohalogenation):
Here, a hydrogen halide is added to the alkyne, and partial and total addition can also take place. An important aspect of this type of reaction is that it follows the Markovnikov's rule, that is, hydrogen binds to the more hydrogenated carbon (with more hydrogen atoms attached) and halogen binds to the less hydrogenated carbon.
In total hydrohalogenation, a gemic dihalide is formed, that is, a compound that has two halogen atoms attached to the same carbon.
Do not stop now... There's more after the advertising ;)
Watch:
HBr Hbr
││ ││
H ─C ≡ C CH3 + HBr → H ─ C ═ C CH3 + HBr → H ─ C ─ C CH3
││
Hbr
ALCINO HALIDE GEMIC DI-HALOGIDE
An important example of this type of reaction is the one that occurs when hydrogen chloride is added to ethyne, forming the chloroethene or vinyl monochloride, which is the monomer that forms the polyvinyl chloride polymer, better known by its acronym PVC.
HClHCl
││ ││
H ─C ≡ C ─ H + HCl → H ─ C ═ C ─ H + HCl → H ─ C ─ C ─ H
││
HCl
ETHINO CHLOROETENE 1,1-DICHLOROETHANE
(PVC monomer)
PVC is a substance widely used in industry for the manufacture of various products, such as sandals, medicine bottles, medical devices, plastic pants for babies, bags, wire coatings, toys, furniture upholstery, car upholstery, raincoats, plastic shoes, vinyl records, floors, packaging films, pipes used in water pipes and sewage etc.
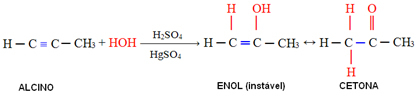
4. Addition of water (hydration):
In this reaction, water reacts with alkyne, initially forming an enol, which undergoes a molecular rearrangement and turns into aldehyde (in the case of alkynes smaller than acetylene). Enol and aldehyde remain in dynamic equilibrium with aldehyde predominance. This is a case of Dynamic Constitucikonal Isomery or Tautomery.
Example:

In the case of alkynes larger than acetylene, the Markovnikov rule is followed and enol gives rise to a ketone:
By Jennifer Fogaça
Graduated in Chemistry