Usually the reactions of aqueous electrolysis and igneous electrolysis they are carried out with graphite and platinum electrodes, which do not participate in the reaction; they only conduct electrical current. However, there are some specific cases where it is necessary to use active electrodes, that is, that undergo reduction and oxidation.
The main use cases of these electrodes that participate in the redox process are: a electrolytic metal purification, such as zinc, cobalt, nickel and especially copper; and also the electroplating. Let's see how each case occurs:
• Copper Electrolytic Purification: this electrolytic copper refining can produce a purity of about 99.9% and is mainly used to make copper wires which, if they have the least amount of impurity, may have their ability to conduct very electrical current diminished.
Observe how this process takes place, seeing the schematic of an electrolytic vat assembled below:

A potential difference is applied to the Copper Sulfate aqueous solution (CuSo
4(aq)) – which conducts electricity – so that the anode, which is an impure metallic copper, loses electrons, that is, it undergoes oxidation, releasing its cations (Cu2+), which are deposited on the negative plate. This can be an inert platinum plate, or, better, a pure copper plate. It constitutes the cathode, which is reduced as copper is deposited in it. Thus, the semi-reactions that occur in the electrodes are:Do not stop now... There's more after the advertising ;)
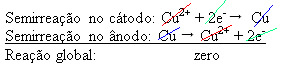
The fact that it gave a zero result indicates that there was no chemical reaction, but only the transport of copper. At the bottom of the container are the other impure substances that were in copper such as gold, silver, silica (sand) and other minerals, which can even be sold.
• Electroplating: a metallic coating is applied to a material that is to be protected from corrosion.
The metal chosen for the coating - which can be chromium (chrome plating), nickel (nickel plating), silver (silver plating), gold (gilding) or zinc (galvanization) – will be the anode, positive electrode, which undergoes oxidation, losing electrons. It undergoes oxidation in place of the material that has been protected, because its reduction potential is greater.
The negative electrode, that is, the cathode, which receives these electrons and undergoes reduction, is the material itself that was coated. In this way, it remains protected even if its surface is somehow violated.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Electrolysis with Active Electrodes"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/eletrolise-com-eletrodos-ativos.htm. Accessed on June 28, 2021.
