Oxides are binary compounds, that is, formed by just two chemical elements, the most electronegative of which is oxygen. See below some of the most important oxides present in our daily lives:
- Calcium Oxide (CaO):
This compound is a white powder known as quicklime or quicklime which, when reacted with water, gives rise to calcium hydroxide (Ca(OH)2), known as quenched lime, slaked lime or hydrated lime. This base is used for whitewash-type paintings on walls, protecting it from infiltration, and on trees to repel insects.

Calcium oxide is widely used in agriculture to correct the pH of acidic soils. However, it is necessary to be careful with the way this application is carried out, because the CaO reacts with the water present on our skin and produces burns. In addition, it also causes damage to the respiratory tract and can cause blindness.
Other applications of CaO are: in constructions, in the preparation of mortar, cement and ceramics; in the manufacture of metallurgical bricks, in the treatment of water and sewage, as an insecticide and fungicide, in the purification of sugars, vegetable oils and fruit juices, in the production of glass, of Na
2CO3 and CaCl (ClO).- Magnesium Oxide (MgO):
MgO is a widely used white powder mixed with water, forming a solution known as milk of magnesia. It is used as a stomach antacid as it reacts with the hydrochloric acid in our stomach and neutralizes the environment.

- Silicon Oxide (SiO2):
the SiO2 it is known as silica and is present in sand and is also found in crystalline form, such as quartz, topaz and amethyst.

The silica from the sand is used in the production of glass, together with the soda ash (Na2CO3) and limestone (CaCO3). When these compounds are heated to a temperature of 1500ºC, a mixture of sodium and calcium silicates is formed, which is cooled to form the glass we know.
Do not stop now... There's more after the advertising ;)
At2CO3 + CaCO3 + SiO2 → sodium and calcium silicates
ash + limestone + sand → glass

- Carbon monoxide (CO):
Carbon monoxide is a polluting and extremely toxic gas that is released in incomplete combustion. Exposure to this gas can occur through pollution caused by the burning of fossil fuels, such as petroleum products, and cigarette smoke.

CO is also used in steel mills to produce metallic iron, reducing iron oxide III from hematite.
- carbon dioxide (CO2 – known as carbon dioxide):
He is one of the main responsible for environmental problems such as the greenhouse effect, global warming and acid rain. It is released in complete combustion of fuels that contain carbon in their constitution and also by our breathing.
This is the gas present in soft drinks and carbonated water. In solid state, it is called dry ice and due to its sublimation property, ie, passing directly from solid to gaseous state, it releases a white smoke that is often used in concerts, theaters, parties and movies.
- Hydrogen peroxide (H2O2):
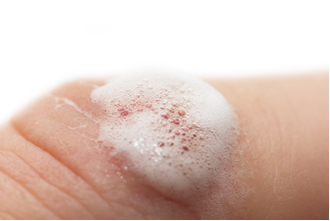
In aqueous solution, hydrogen peroxide is called hydrogen peroxide and is used (at 3%) as an antiseptic and bleach. In higher concentrations, it is used to lighten hair and hair; and in concentrations above 30%, it is used in industries such as wood bleach, textile fibers and in rocket propulsion.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Main Oxides of Daily Life"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/principais-Oxidos-cotidiano.htm. Accessed on June 28, 2021.
Chemistry

Binary compounds, peroxides are used as clarifiers, fabric bleaches, cellulose pulp, mortar preparation, quicklime, dry ice, hydrogen peroxide, hydrogen peroxide.
Chemistry

Nitric oxide, lipophilic, synthesized by endothelial cells, gaseous free radical, processes intracellular and extracellular, Hemodilation, widening of blood vessels in muscles, supplements food.