High school students are always subject to facing redox reactions with ions, both in entrance exams and in the National High School Examination (Enem). Thus, balancing these equations can be a much dreaded subject matter.
In order to facilitate the understanding of this subject, this text seeks to address the balancing of redox equations with ions with the description of some steps to carry it out. For this, we must start by identifying this type of chemical reaction.
At redox reactions with ions are identified by the presence of one or more components with charges (positive or negative), that is, the ions (cations or anions), which can be present in reagents or products, as in the two examples a follow:
1st Example:Cr2O72- + Fe2+ + H+ → Cr3+ + Fe3+ + H2O
2nd Example:Cl2 + C + H2O → CO2 + H+ + Cl-
To carry out the balancing equations of redox reactions with ions, it is essential to follow some steps, such as:
1st Step: determine the NOX of each of the atoms of the reactants and products of the equation;
2nd Step: check which species suffered oxidation;
3rd Step: determine the variation of the NOX the species that has undergone oxidation;
4th Step: multiply the variation found in the third step by the number of atoms of the oxidizing species;
5th Step: check which species suffered reduction;
6th Step: determine the NOX variation of the species that underwent reduction;
7th Step: multiply the variation found in the third step by the number of atoms of the species that it reduces;
8th Step: use the value found in the fourth step as the coefficient of the species that reduces;
9th Step: use the value found in the seventh step as the coefficient of the oxidizing species;
10th Step: put the coefficients in the other species, according to the method of trial balancing. In those species where it is not possible to do this, put unknowns as coefficients;
11th Step: multiply the coefficient of each species by its load;
12th Step: Equalize the sum of charges of reactants with that of products to find the coefficient of one of the species with unknowns;
13th Step: finalize the balancing using the trial method.
In order to facilitate understanding, follow the step-by-step described above, in the following example of a redox equation with ions:

Ionic equation showing some ions and general charge groups 0
1st Step: NOX of each atom.

NOX of atoms present in the equation
2nd Step: Species that undergo oxidation.

Species that undergo oxidation in the equation
As the NOX of iodine increases from -1 to 0 from reactant to product, it is therefore the species that undergoes oxidation.
3rd Step: Determine the NOX variation of the oxidized species.
Do not stop now... There's more after the advertising ;)
To do this, just subtract the highest NOX by the lowest NOX:
∆NOX = (0) – (-1)
∆ NOX = 0 + 1
∆ NOX = 1
Step 4: NOX multiplication by quantity.
In this step, we multiply the variation found in the third step by the number of atoms of the species.
I- = ∆NOX.1
I- = 1.1
I- = 1
5th Step: Species that undergo reduction.

Species that suffer reduction in the equation
As chromium NOX decreases from +7 to +3 from reactant to product, it is therefore the species that undergoes the reduction.
6th Step: Determine the NOX variation of the reduced species.
To do this, just subtract the highest NOX by the lowest NOX:
∆NOX = (+6) – (+3)
∆ NOX = +6 – 3
∆ NOX = 3
7th Step: NOX multiplication by quantity.
In this step, we multiply the variation found in the sixth step by the number of atoms of the species.
Cr2O72- = ∆NOX.1
Cr2O72- = 3.2
Cr2O72- = 6
8th Step: Start balancing.
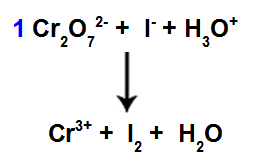
Start of balancing using found coefficient
In this step, we start the balancing by placing the coefficient found in the fourth step in the species that suffered reduction.
9th Step: Continue balancing.

Continuation of balancing using the found coefficient
10th Step: Use the trial method.
There are 6 I atoms in the reactant, so we must put the coefficient 3 in the I species2 of the product, because there are only 2 Cl atoms in it;
As in species 1, Cr2O72-, there are 2 Cr atoms, we should put the coefficient 2 in the Cr species3+ of product.
As we have oxygen in three species, and in two of them there are no coefficients, to continue the balance, we must place unknowns in the remaining species:
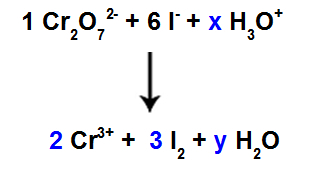
Coefficients after using the trial method
11th Step: Multiplication of each species by its load.
To do this, just multiply the coefficient of the species by its load, as follows:
In the reagents:
Cr2O72- = 1.(-2) = -2
I- = 6.(-1) = -6
H3O+ = x.(+1) = +x
On the products:
Cr3+ = 2.(+3) = +6
I2 = 4.0 = 0
H2O = y.0 = 0
12th Step: Sum of charges.
In this step, we must add the loads of reagents and products found in step 11:
Reagents = products
-2 + (-8) + (+x) = +6 + 0 + 0
-2 - 6 + x = 6
-8 + x = 6
x = 6 + 8
x = 14
13th Step: End of balancing.
To complete the balancing, we must:
Put the result found in the 12th step in species H3O+;
As we now have 42 H atoms in the reagent, it is necessary to place the coefficient 21 in the H species2The in the product.
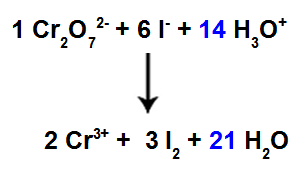
Balanced Ionic Reaction Equation
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Red-Reduction Reactions with Ions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-oxirreducao-com-ions.htm. Accessed on June 28, 2021.