Sensitive heat or sensitive specific heat is a physical quantity that is related to the variation in the temperature of a body.
Example: Heating a metal bar
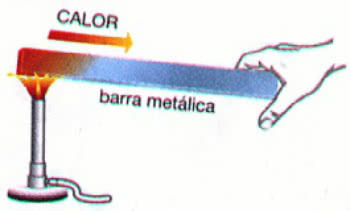
In the example above, heat propagates through the thermal conduction. This process results in an increase in the temperature of the material, however, its physical state remains the same (solid).
Formula
To calculate the sensible heat, use the following formula:
Q = m. ç. Δθ
Q: amount of sensible heat (lime or J)
m: body mass (g or kg)
ç: specific heat of the substance (cal/g°C or J/Kg°C)
Δθ: temperature variation (°C or K)
Note: To calculate the sensible heat we have to know the specific heat that varies in each substance.
Read more at: Specific heat.
Sensitive Heat and Latent Heat
At the latent heat (L), the physical state of the substance is modified, while in sensible heat it remains the same.
Another difference between the two is regarding temperature. That is, latent heat is independent of body temperature, while sensible heat considers it.
An example of latent heat is the melting of an ice cube or the evaporation of water. In both cases, the temperature in the two physical states remains the same.
To calculate latent heat, use the following formula:
Q = m. L
Where,
Q: amount of heat (lime or J)
m: mass (g or kg)
L: latent heat (cal/g or J/Kg)
Read too:
- Heat and Temperature
- Calorimetry
- Thermal balance
- Thermal Capacity
- Physics Formulas
Entrance Exam Exercises with Feedback
1. (Mackenzie) A heat source supplies heat continuously, at the rate of 150 cal/s, to a given mass of water. If the water temperature increases from 20°C to 60°C in 4 minutes, with the specific heat sensitive of the water being 1.0 cal/g °C, it can be concluded that the mass of heated water, in grams, is:
a) 500
b) 600
c) 700
d) 800
e) 900
Alternative and
2. (UFSM-RS) A 400 g body and sensitive specific heat of 0.20 cal/g °C, at a temperature of 10 °C, is placed in thermal contact with another 200 g body and sensitive specific heat of 0.10 cal/g° C, at a temperature of 60°C. The final temperature, once the thermal balance between the two bodies is established, will be:
a) 14°C
b) 15°C
c) 20°C
d) 30°C
e) 40°C
Alternative c
3. (UFPR) During the eclipse, in one of the cities in the totality zone, Criciúma - SC, there was a drop in temperature of 8.0°C. (Zero Hours – 11/04/1994)
Knowing that the sensitive specific heat of water is 1.0 cal/g °C, the amount of heat released by 1000 g of water, when reducing its temperature from 8.0 °C, in lime, is:
a) 8.0
b) 125
c) 4000
d) 8000
e) 64000
Alternative