Before we understand what the magnetic quantum number and the quantum number are all about spin, it is necessary to remember what the word orbital means.
Orbital is the region of space around the nucleus where the probability of finding a particular electron is greatest.
To better understand, think of a hive. It is not possible to say with certainty the trajectory and position of each bee, however, we can indicate the region around the hive where the bees are most likely to be found. Similarly, the orbital indicates the region around the nucleus of the atom where the position of the electrons will be most likely to be determined.
Since the electron has a dual characteristic, that is, it behaves as a particle and also as a wave; scientists prefer to identify it according to its energy content. Through mathematical calculations, the scientist Erwin Schrödinger, in 1927, related the corpuscular nature, energy, charge and mass of the electron. Thus, each electron came to be characterized by quantum numbers.
Quantum numbers are mathematical codes associated with electron energy.
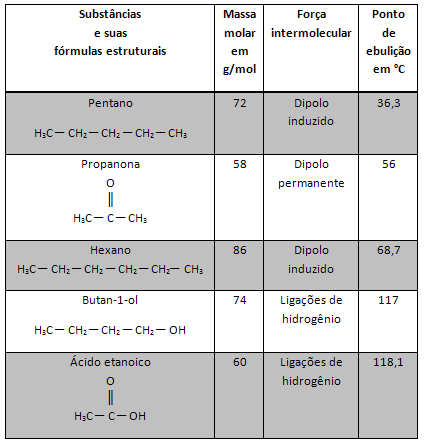
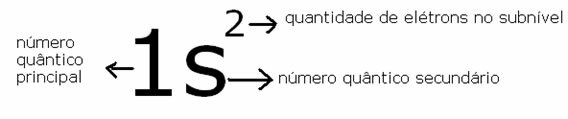
Two best known quantum numbers are the principal quantum number (n) and the secondary or azimuthal (there).The main one indicates the layer or energy level (K, L, M, N, O, P, Q) in which the electron is found, going, respectively, from 1 to 7. The azimuthal represents the energy sublevels (s, p, d, f), which are, respectively, 0.1,2,3. Both appear in the energy diagram below, where the main quantum number represents the seven “stairs” and the secondary quantum number is represented by the “stairs” of the stairs.
Now let's consider the magnetic quantum number and the spin:
- Magnetic Quantum Number (m or mthere) → indicates the orientation of the orbitals in space.
According to the energy diagram below, the values of m are represented by small squares (or by balls, depending on the author). For each orbital we have a value for the magnetic quantum number, which varies from –there The+1.
Do not stop now... There's more after the advertising ;)

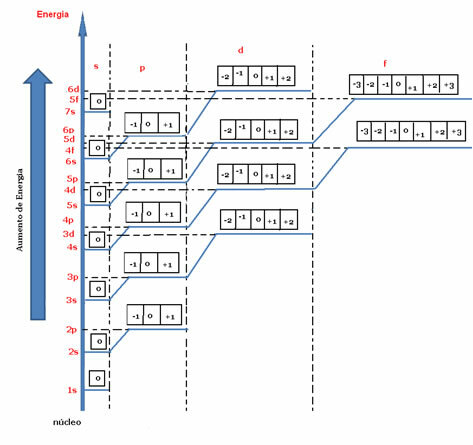
Energy diagram indicating the magnetic quantum number
-
Quantum Number spin (s or ms)→An orbital holds a maximum of two electrons. They don't repel each other because they rotate in opposite directions, creating magnetic fields that attract each other. So, the force of attraction, which is the magnetic; and that of repulsion, which is electrical, are counterbalanced.
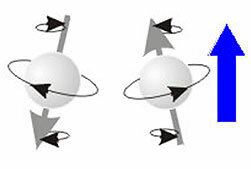
Opposite or antiparallel spins, electrons spinning in opposite directions.
In this way, we represent in each small square only two electrons at most, which are represented by arrows and have the values of +1/2 and -1/2.
↑ |
It represents, by convention, an electron with negative spin s = -1/2.
↓ |
It represents, by convention, an electron with positive spin s = +1/2.
As an example, let's look at the element helium, which has two electrons and only one energy level; its symbol is: 2he
↑↓ |
Its symbolic representation is given by:
Representation of the position of the most energetic electron in Helium
So, we have their quantum numbers for the 1st and 2nd electron:
1st electron: 2nd electron:
n=1 n=1
l= 0 l=0
m = 0 m = 0
s= -1/2 s= +1/2
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Quantum numbers: magnetic (m or ml) and spin (s or ms)"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/numeros-quanticos-magnetico-m-ou-ml-spin-s-ou-ms.htm. Accessed on June 28, 2021.