Have you ever been bothered because you went to butter your bread, but since it was in the fridge, it was very hard and difficult to slide? This has happened to a lot of people, and that's why they started leaving butter out of the fridge. This is even understandable, considering that the consistency of the food is one of the most important factors in flavor perception during chewing and is something even taken into account by industries food. Others do this because the butter needs to be softer when preparing certain foods.
But then a question arises: butter should be stored inside or outside the refrigerator?
One important thing you need to understand that determines how to store butter is that if you leaving the butter out of the fridge and consuming it could pose an imminent risk to your health!
To understand why this is so, let's first look at the chemical composition of butter. butter is a fat, this means that she is part of a group of lipids, also calledglycerides, which are compounds formed by the union of three
fatty acids (carboxylic acids long-chain, usually with 4 to 22 even-numbered carbon atoms, with only one carboxyl group) and a molecule of the glycerol, which is a glycerin (propanetriol) trialcohol molecule, as shown below: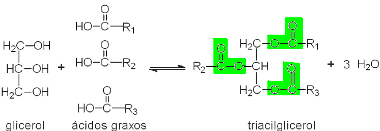
The formation of a glyceride occurs by the reaction between a glycerol and fatty acids.
Note that there are three ester groups in green. Therefore, glycerides are also called triglycerides. If the fatty acid radicals that formed the compound do not have double bonds, that is, if they are saturated, fats (solids) are formed, otherwise, oils (liquids) are formed. This formation of fats can occur in vegetables and animals. In the case of butter, it is of animal origin, as it is derived from cow's milk.
The butter production process basically consists of obtaining, with a creamer, a cream, a high-fat dairy product in the form of an emulsion in water. This cream goes through a standardization process so that the fat content is between 38 and 42%, then it is pasteurized at 85ºC to eliminate microorganisms. Afterwards, it goes to a continuous mixer to separate the fat from the buttermilk, goes through a process of washing and kneading or kneading to make the mass more homogeneous and also to regulate its consistency and structure. Artificial color and salt can be added.
Thus, butter is basically formed by this cream which has, on average, the following fatty acids in its percentage composition:

Average percentage composition of fatty acids in butter.
Note that the fatty acid that has the highest percentage in butter is linoleic acid (omega-6 and that its melting point is -5°C). Because it is rich in saturated fats, butter tends to solidify at lower temperatures because of its close-to-linear arrangement. But see below the structure of linoleic acid, it has two unsaturations. When the material is composed only of saturated fatty acids, it has a very high melting temperature and becomes unsuitable in relation to the desired consistency for this type of product. Therefore, butter is semi-solid, as it has mostly saturated chains, but it also has less unsaturated acids.
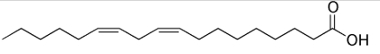
Linoleic acid structure.
Due to this composition, if we leave the butter out of the refrigerator, the contact with the humidity of the air, with oxygen and with high temperatures it will result in the proliferation of microorganisms, such as fungi and bacteria, which will cause a very complex type of reaction, called rancification. In the case of butter, the oxidative rancidity, which occurs as follows: when butter is stored improperly, that is, if it is not inside the refrigerator, the double bonds of fatty acids unsaturated substances form free radicals that react with oxygen (oxidation reaction) and form products that change the characteristics of the lipids.
will then occur the chain break of these mentioned glycerides and rancid-smelling acids will be formed. The main acid that causes this odor is butyric acid (H3C ─ (CH2)2 ─ COOH). Even his name comes from the Latin butyrum, which means "butter" precisely because it gives off the peculiar odor of the rancidity of butter.
This oxidative racification is benefited by the characteristic factors of the environment outside the refrigerator, such as: greater amount of oxygen, direct exposure to light and the increase in temperature that increases the speed of oxidation (to give you an idea, a 10°C increase in temperature will double the reaction of oxygen with the fat). The presence of water (moisture) and metals that become catalysts also accelerate the reaction. Even the presence of water favors another type of rancidity, which is the hydrolytic rancidification, in which the ester bond is hydrolyzed by the action of enzymes, such as lipase, or by a chemical agent in the presence of moisture, also releasing saturated and unsaturated free fatty acids.
The type of fatty acid that undergoes rancidity can also accelerate this reaction. For example, the linoleic acid mentioned above oxidizes 64 times faster than oleic acid, which is monounsaturated:
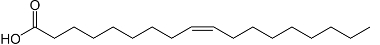
Structure of oleic acid.
Linolenic acid (omega-3) oxidizes 100 times faster because it has three double bonds:
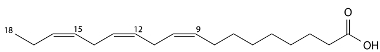
Linolenic acid structure.
So, for all these factors, leaving butter out of the refrigerator causes this reaction that changes the shape, color, flavor, texture and odor of the butter, not that's all, it also reduces its nutrients and makes it unfit for human consumption, potentially causing food poisoning.

Consuming butter left out of the refrigerator for too long can cause food poisoning.
But, on average, consumption of butter left out of the fridge becomes dangerous after about two hours.
So, do you understand how to store butter? Of course the correct thing is inside the fridge. But, what can you do to consume a softer and safer butter? Here are some tips:
* You may take the butter out of the fridge about half an hour before using it, but then save it back immediately;
* If your time is shorter and you can't wait 30 minutes, cut the margarine, because the larger the contact surface area, the faster it will soften.

Sliced butter to soften faster.
* use the microwave at 30% potency and keep looking every 5 seconds until you reach the desired consistency;
Note: This risk of poisoning also applies to other dairy foods, such as cheese, yogurt and milk itself. Therefore, only remove them from the refrigerator at the time of consumption.

Milk derivatives must be kept refrigerated.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/manteiga-fora-ou-dentro-geladeira.htm