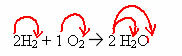Chemistry is a science that is organized into several branches, one of which is that of Analytical chemistry, an area that studies and applies techniques for identifying samples, which can be natural or artificial.
Analytical Chemistry, in turn, is also subdivided into some fields, according to the study objective. If this objective is to discover exactly which elements make up a (qualitative) sample and in what proportion they appear in the molecule or in the formula (quantitative), then it is the branch of elementary analysis.
For example, let's say a colorless liquid substance was found at a crime scene. Knowing what this substance is can help you find out how the victim was killed and who the murderer was, depending on the case. This sample found may be just a substance, such as water, or a mixture of substances, such as water and alcohol.
Thus, before proceeding to quantitative or qualitative study, the chemist first analyzes physical and chemical properties of the material.
For example, if the sample is a pure substance, it will have a fixed boiling point and melting point at a certain temperature each. On the other hand, if it is a mixture, the melting and boiling points will not be fixed and constant, but the change of physical state will occur over a range of temperatures.
If the sample found at the crime scene mentioned above has a fixed boiling point at 100°C and a fixed melting point at 0°C, the chemist will already know that it is water. But let's say it's actually a mixture, so it's going to use mixture separation techniques, such as precipitation, extraction and distillation. For example, if each component (analyte) has a different boiling point, a distillation can be carried out.
To find out which elements make up the substance's formula or molecule, the chemist begins to perform the qualitative elementary analysis, in which decomposition reactions and standardized tests are performed, such as treating analytes with reagents that can produce compounds that can be identified by color, solubility, melting and boiling points etc.
Do not stop now... There's more after the advertising ;)
For example, the substance can be dissolved in bases or acids to check for color changes or precipitate formation to identify the starting substance.

To also discover the proportion in which the elements that make up the substance appear in its formula or molecule, the chemist proceeds with the techniques of quantitative elementary analysis. This determination is usually made initially in mass or volume and then in quantity of matter (mol).
Some commonly used techniques are volumetry (titrations) and gravimetry (mass measurements). These classical methods are widely used due to the relative simplicity of the equipment needed and the reliability of the results obtained.
Currently, however, there are many modern analytical equipment that have or are connected to one or more sophisticated electronic devices, such as amplifiers, integrated circuits, microprocessors or even computers, which are capable of performing both qualitative and quantitative analysis directly. This is very important not only for the precision and accuracy of the analysis, but also for preventing the analyst from exposing himself to dangerous substances, such as gases that could poison him.

JSM-6510 Scanning Electron Microscope at the International Exhibition of Analysis and Laboratory Equipment, April 28, 2011 in Moscow*
With this, it is possible to determine the molecular mass of the substance and its percentage, minimum and molecular formulas, which allows to identify which substance it is.
* Editorial credit: dikiiy / shutterstock.com
By Jennifer Fogaça
Graduated in Chemistry