According to the First Law of Thermodynamics, in any thermodynamic process the amount of heat Q received by a system is equal to the work done by it plus the variation of its internal energy.
When pressure is constant, the heat exchanged by the system with the external medium is used for work and to change the internal energy. In many practical situations, systems are subjected to atmospheric pressure, as in the case of a chemical reaction. The figure above shows the PV diagram of this type of process.
In this case, in the equation of the First Law,
Q=τ+∆U
none of the terms are zero. The work is written as a function of volume variation volumeV, such as:
τ=P.∆V
For the particular case of an ideal monoatomic gas, the energy can be written as follows:

Therefore, we can write the First Law of Thermodynamics as a function of ΔV:


The heat exchanged with the medium is (5/2)P.ΔV, and 40% of the total – which corresponds to P.ΔV – is used to perform work; and (3/2)P.ΔV, which corresponds to 60% of the total, are used to alter the internal energy. This result is valid for an ideal monoatomic gas.
Heat is related to temperature variation (using the ideal gas law) by:
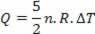
Thus, the heat supplied can be calculated by the change in temperature or by the change in volume.
By Domitiano Marques
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/fisica/primeira-lei-para-processos-isobaricos.htm

