Heatlatent is the amount of energythermal which is absorbed or given up by a body or thermodynamic system, during a change in its physical state, in constant temperature.
When a pure substance reaches its temperature of Fusion or boiling, during your warm up, your physical state starts to change. In this process, it continues to absorb heat, however, its temperatureremainsconstant. This happens because, when reaching these temperatures, in which physical state changes occur, all the heat that is being absorbed by the thermodynamic system is used to overcome the energypotential that keeps its molecules together. As soon as the thermodynamic system absorbs all the energy necessary to break up its molecules, the interaction between them decreases, indicating that their state of aggregation has changed. After the change in physical state, the heat that was absorbed isothermally continues to be absorbed by the molecules, providing them energykinetics. This type of heat that increases the kinetic energy of molecules is called sensible heat.
Lookalso: Seven “Gold” Tips for a More Effective Physics Study
O heatlatent measures the amount of heat, per unit of mass, needed for any change in physical state of the body, therefore, its unit of measure, according to the International System (SI), is the Jouleperkilogram (J/kg). However, the use of other units such as the caloriepergram (cal/g), is quite common in the study of calorimetry.
Types of latent heat
There are two types of heat: o heatiflevel it's the heatlatent. Sensitive heat is that which is transferred between bodies when there are changes in temperature. Latent heat, in turn, occurs when there are heat transfers without temperature changes.
Latent heat is altered for different changes in physical state. Check out the different types of latent heat:
latent heatinmerger (LF): it is the heat that is absorbed or given up by the bodies during the fusion process: from liquid to solid and vice versa, with constant temperature.
latent heatinvaporization (LV): is the one that is transferred during solid-liquid or liquid-solid transformations, in constant temperature.
Lookalso: What is thermal capacity?
Examplesinheatlatent
Check out some everyday situations in which there are latent heat exchanges:
When we heat the water, up to a temperature of 100°C, it starts the evaporation process. As long as all the water doesn't turn into vapor, its temperature doesn't change.
When we pour water onto a very hot surface, all the water vaporizes almost instantly. This process is called heating and involves the absorption of latent heat.
There is latent heat exchange when we touch a soda bottle at low temperatures and all its contents freezes quickly at a constant temperature, thanks to its temperature lower than the melting point of water.
latent heat formula
Latent heat is calculated by the ratio of the amount of heat transferred in the isothermal transformation:
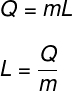
Q – amount of heat transferred
m – body mass
L – latent heat
Phase changes and latent heat
Phase changes in pure substances occur intemperatureconstant, by absorbing or releasing latent heat. All pure substances have a heating curve similar to the picture below:
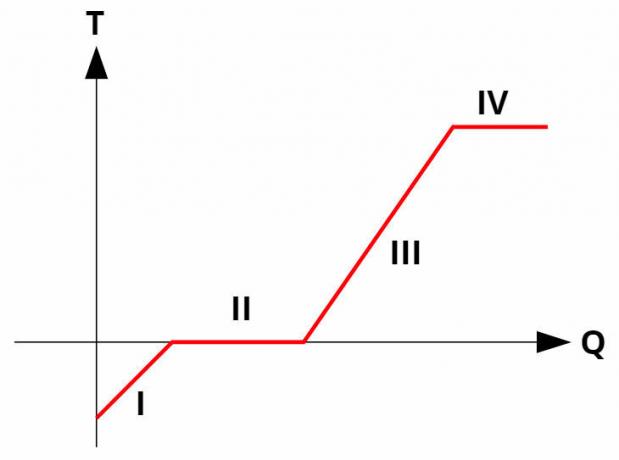
At curvesinheating they relate temperature (y-axis) to the amount of heat given or received (x-axis). In phase changes (processes II and IV), the temperature remains constant, although there is still heat exchange.
See too: Fundamentals of Calorimetry
latent heat table
Under normal conditions of temperature and pressure, O heatlatent of water, for its different changes in physical state, is shown in the table below:
Transformation |
Latent heat (cal/g) |
Fusion (0°C) |
80 |
Solidification (0°C) |
-80 |
Vaporization (100°C) |
540 |
Condensation (100°C) |
-540 |
According to the table shown above, 80calories to freeze 1 gramof water at the melting temperature (0 °C). The negative signs in the processes of solidification and condensation indicate that heat was released in them, so these two transformations are exothermic. The table below shows the latent heat in J/kg, for the same processes:
Transformation |
Latent heat (J/kg) |
Fusion (0°C) |
333.103 |
Solidification (0°C) |
-333.103 |
Vaporization (100°C) |
2,2.106 |
Condensation (100°C) |
-2,2.106 |

latent heat exercises
1) One container holds 500 g of liquid water. With no changes in water temperature, all of its contents are suddenly evaporated. Determine how much heat has transferred to the contents of this container.
Data: LF = 540 cal/g
Resolution:
To calculate the amount of heat needed to evaporate this mass of water, we will use the following formula:
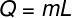
Using the data provided by the exercise, we will do the following calculation:
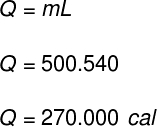
By Me. Rafael Helerbrock


