The phenomenon of radioactivity caught the attention of numerous scientists, including the New Zealand physicist Ernest Rutherford (1871-1937). He performed an experiment in which a beam of alpha particles (α) was subjected to an electric field. Rutherford noted at the end of the experiment that this radiation would be formed by positive particles, since it was attracted by the negative pole.
He also found that there were negative particles that were attracted by the positive pole; these were the beta (β) particles.Furthermore, this radiation had a higher penetration power than alpha radiation.
However, there was one of the radioactive emissions, the range (γ), who was not attracted to any of the poles. This is even more energetic than other radiations. Therefore, it was concluded that gamma radiation (γ) is not made up of particles, but, like X-rays, it would be formed by electromagnetic waves, in addition to having no charge or mass. As it has no charge, this radiation does not suffer interference in the electric field.
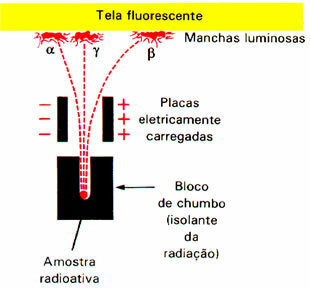
An experiment carried out by Rutherford detected that the alpha and beta particles were deflected by the electromagnetic field.
This and other later studies demonstrated that Dalton's atomic model, in which the atom would be a sphere, massive and indivisible, could not be correct; for, as seen above, the atom should have smaller particles with positive and negative charges.
In 1911, Rutherford proposed that the atom would be composed of an atomic nucleus, in which would be the positive particles, called protons; and in the electrosphere, that is, in the region around the nucleus, the negative particles (electrons) would be rotating in circular orbits.
He himself later found that radioactivity was a phenomenon that occurred in unstable atomic nuclei.
Physicists F. Soddy, A. Russell and K. Fajans, independently of each other, discovered which were the corresponding parts of these radiations within the atom:
*Alpha particles (α):When emitting an alpha particle, the radioactive element atom is actually emitting two protons and two neutrons (the positive charge is due to the protons);
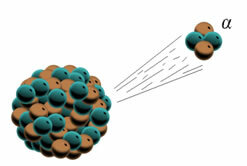
When an element emits an alpha particle it is emitting two protons and two neutrons.
*Beta particles (β): When a radioactive element emits a beta particle, it is losing an electron and a subparticle called an antineutrino. A neutron decomposes, giving rise to a proton that remains in the nucleus, an electron and an antineutrino that are emitted.
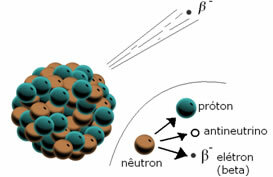
When an element emits a beta particle, it is emitting an electron.
Thus, the characterization of these three types of radiation is given below:
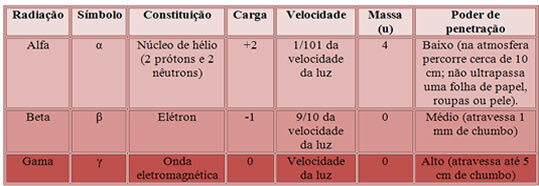
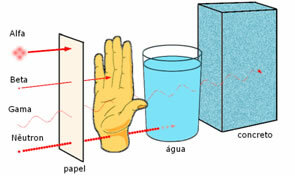
Penetration power of the three main nuclear radiations.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team.
Source: Brazil School - https://brasilescola.uol.com.br/quimica/radioatividade-estrutura-atomo.htm
