THE ozone layer, as the name implies, is a layer or coating formed by ozone gas molecules (O3(g)), an allotropic form of oxygen whose molecule is shown below:
It is located in a layer outside the atmosphere, at an altitude between 20 and 35 km, being called the stratosphere. But this gas can also be found in smaller quantities in the troposphere (altitude about 10 km).

The ozone layer plays a vital role in the maintenance of life on Earth, as it is able to absorb up to 99% of the ultraviolet (UV) radiation from the Sun. Since this radiation has short wavelengths and high energy, it has high skin penetration power. It is this radiation that causes tanning, but it is also responsible for many harmful effects, as it can damage DNA (deoxyribonucleic acid), causing genetic mutations.
UV radiation is divided into three distinct energy ranges: UVA (320 nm to 400 nm), UVB (290 nm to 320 nm) and UVC (200 nm to 290 nm). Among them, the most harmful and energetic is UVC, which, fortunately, does not reach the earth's surface because it is filtered by the layer. of ozone.
So, the ozone layer is really a versatile and efficient shield that helps to protect from this harmful radiation. many forms of life, such as plankton, which are responsible for producing much of our oxygen.
The amount of ozone in the stratosphere is not constant, but it is directly proportional to the intensity of UV radiation. The formation of molecules of this gas occurs through the decomposition of oxygen gas molecules (O2(g)), forming free oxygen that reacts, in a second step, with the oxygen gas:
1st step: The2(g) → 2 O(g)
2nd stage: The(g) + O2(g) → 1 The3(g)
A chemical balance is then formed in the ozone layer:
2 O2(g) ↔ 1 O3(g) + O(g) ∆H = + 142.35 kJ/mol
Unfortunately, however, over time, human beings have released some pollutant compounds that shifted this balance towards the decomposition of the ozone, decreasing its concentration in the stratosphere and leaving the planet more unprotected.
One of the biggest causes of the destruction of the layer of Ozone are CFCs (Chlorofluorocarbons, also known as Fréons®), which are compounds formed by carbon atoms, fluorine and chlorine. CFCs are released into the atmosphere primarily through their use as an aerosol propellant (sprays), in refrigerators and refrigerators, as an expanding agent for plastics and in solvents to clean electronic circuits.

As you can see from the reactions below, when CFC hits the stratosphere, solar radiation breaks down its molecules, releasing chlorine. Chlorine, in turn, reacts with ozone and this reduces its concentration:
CH3Çℓ(g) → CH3(g)+Çℓ(g)
Çℓ(g) + O3(g) → CℓO(g) + O2(g)
In addition, the CℓThe formed also reacts with the free oxygen atoms in the atmosphere, releasing more chlorine atoms, which will react with the ozone, increasingly destroying our protective layer:
ClO(g) + O(g) → Cl(g) + O2(g)
The worst affected place is Antarctica, where the hole in the ozone layer was twice as big as in Europe in September 2000. NASA's ozone monitoring satellite recorded the largest hole ever seen over Antarctica, measuring about 28.3 million square kilometers, which represents more than three times the area of Australia. This situation is worse in Antarctica because there the formation of chlorine atoms is very large and remains unchanged, due to the atypical stratospheric clouds formed during the austral winter, and it is on the surface of the particles of these clouds that the reactions occur shown.
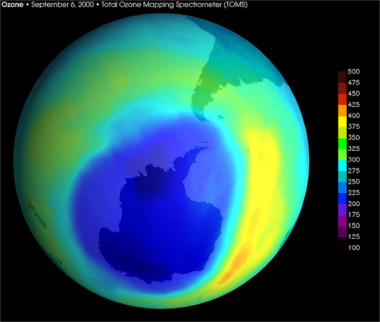
NASA satellite image of “hole” in the ozone layer over Antarctica, September 2000
The possible consequences of the destruction of the ozone layer are theincrease in the incidence of skin cancer, due to the action of ultraviolet rays, and the intensification of global warming, the which leads to several catastrophic results, such as the melting of polar glaciers, raising the water level in the oceans.
But there is still a glimmer of hope, since since 2000 the concentrations inCFCs have declined by nearly one percent a year.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/camada-de-ozonio2.htm
