Osmosis is the passage of solvent through semi-permeable membranes. It can occur between a solution and a pure solvent and between two solutions. In the first case the pure solvent passes through the membrane towards the solution. In the second case, the solvent of the more dilute solution passes to the more concentrated solution.
It is this last example that occurs in plant roots. Within them there is a solution more concentrated than the land around them. In this way, through osmosis, the solvent passes through the earth, that is, water, together with some mineral salts, into the plant.
A semipermeable membrane is that selective membrane, that is, one that only allows certain substances to pass through. In the case of plants, the cell membrane acts as the semi-permeable membrane, as it allows substances such as water and urea to pass through. However, other substances do not pass through it, such as glucose and sodium ion.
Furthermore, osmosis always occurs in the sense of a high chemical potential to a low chemical potential. In a plant, the root is the region of high chemical potential; and in leaves this potential is lower. Thus, the movement of water from the root towards the leaves occurs so that photosynthesis occurs.
Vegetables that grow in the soil and act as plant roots also carry out this osmosis process. So you can check this out, do a simple experiment*: take a carrot and make a hole in the middle along its length. Fill part of this hole with a concentrated sugar solution and mark the volume of the solution on the carrot with a ballpoint pen. Afterwards, dip the carrots into a bowl of water.
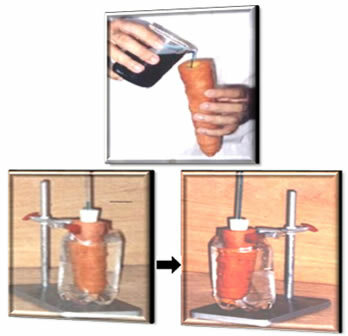
After a few days you will notice that the water volume has risen, proving that there has been osmosis from the outside to the inside of the carrot.
*For more details about this experiment, see the text “Carrot Osmosis - Experimental Activity”.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/osmose-nas-plantas.htm

