Is glass solid or liquid? Have you ever heard this question? This question has puzzled people for a long time. The first studies on the structure and definition of glass were carried out in 1830, and since then many concepts have been changed as science and technology developed.
At first, probably the first answer that would come to mind would be that glass is solid. However, doubts begin to arise when we come across its manufacturing process. Let's understand a little more about this?
There are several methods of glass production, such as chemical vapor deposition, pyrolysis, neutron irradiation, sol-gel process, among others. The most used process today is the classic method of melting/cooling.
As the name implies, in this process, a mixture of powdered substances is taken to an oven, at a temperature of around 1500ºC. In this oven, the mixture melts (goes from solid to liquid) and forms a pasty mass with a viscosity similar to that of honey. This glass is then removed from the melting furnace and shaped as it is cooled, reaching the rigid structure we know.

Handmade Glass Manufacturing Steps
Generally the substances used in the glass mixture as raw materials for the preparation of glass, are the silica or silicon dioxide (SiO2 ), which is present in the sand, but in factories it is customary to use another crystalline form of silicon dioxide, which is quartz; The soda or soda (sodium carbonate - Na2CO3) it's the limestone (calcium carbonate - CaCO3). These three materials are crushed and turned into powder, then mixed in the proper proportion.
The mass formed with the fusion consists of sodium and calcium silicates:
ash + limestone + sand → common glass + carbon dioxide
At2CO3 + CaCO3 + SiO2 → sodium and calcium silicates + carbon dioxide
x In2CO3 + y CaCO3 + z SiO2 → (At2O)x . (CaCO)y . (SiO2)z+ (x + y) CO2
In industries, it is common to add broken glass to the mixture, which is a means of glass recycling.
Although only inorganic substances are mentioned here, there are also glasses made of organic and metallic materials.
In analyzing this process, some might think that the glass would be liquid, as it comes out as a homogeneous liquid after melting in the furnace. However, glass is classified neither as a liquid nor as a solid only, but as a non-crystalline solid. Like this???
To understand, let's compare glasses to crystals. These, in general, are crystalline solids, that is, they present a structure whose arrangement of atoms is periodic and symmetrical.
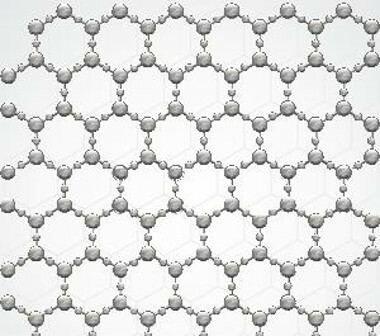
Illustrative representation of crystalline solid with symmetrical and periodic crystalline arrangement
Glass, on the other hand, does not have an atomic arrangement with symmetry and translational periodicity, but is formed by an extended and random three-dimensional network, as shown in the following illustration:

Illustrative representation of the glass network (non-crystalline solid) where the absence of symmetry and periodicity is characterized
Based on this, some claim that glass is an amorphous solid. However, although amorphous solids are also non-crystalline solids, they are different from glasses. While glasses have a glass transition, amorphous solids do not have this phenomenon.
Thus, we can define glasses as non-crystalline solids that have a glass transition. But what is a glass transition?
When the molten glass is being cooled, it takes some time for the forming units to orient themselves so that they are organized and thus form the crystal. This phenomenon occurs in a temperature range called glass transition. This is a range of temperatures that starts with structural relaxation, that is, when they start to changes occur in some material properties, such as viscosity, heat capacity and expansion thermal.
Thus, the glass transition temperature defines the transition from the glassy state to the viscoelastic state. This refers to materials that, when applying a force, respond elastically, but not instantly or permanently. The vitreous state, on the other hand, corresponds to a behavior in which, when we apply a force on the material, it does not respond elastically — it does not deform — but absorbs and dissipates energy. So the result is that the body breaks down.
When this cooling is done quickly (which is the case with glass), the units lose mobility before they organize themselves, and crystallization does not occur. This means that the glass cooling takes place at a temperature below the glass transition temperature. If the temperature is higher than the glass transition temperature (which is characteristic for glasses), the material will have a viscoelastic behavior.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/o-vidro-solido-ou-liquido.htm
