In the text oil refinement it is shown that this organic product is generally not used in raw form. As it is made up of a complex mixture of hydrocarbons, petroleum undergoes a process of refinement in which the petroleum fractions, that is, groups of mixtures that contain fewer compounds and that have similar molar mass in each fraction.
The main difference between these fractions is in the amount of carbon atoms in the molecules of their constituents. The larger this number, the heavier the fraction. For example, the lightest fraction is natural gas, which is made up practically of methane, which contains only one carbon atom. Gasoline, which is liquid, has molecules with 6 to 10 carbon atoms. The oil diesel it has 15 to 18 carbons, and paraffin has high molar mass solids, such as C36H74.
Since the difference is only in the size of the molecules, a chemical process emerged in which some petroleum fractions, as well as petroleum residues that remain after fractionation, are submitted. This process is called
cracking and is also known ascracking. The word "cracking" comes from the English I'm cracking, which means "to break".This is exactly what is done in this process, the breaking of long hydrocarbon molecules of high molar mass to form other molecules with smaller chains and lower molar masses, such as alkanes, alkenes and even carbon and hydrogen.
For example, the kerosene fraction is formed by molecules with 10 to 16 carbon atoms, such as C12H26. This is a long molecule that can go through cracking in oil refineries and be transformed into smaller molecules like C8H18, which makes up gasoline. See this reaction below:
1C12H26 → 1 C8H18 + 2 C2H4
alkene fraction fraction
kerosene gasoline (ethylene)
This process is very important, as it allows fractions of oil that are sold for lower values to be transformed into fractions of greater commercial value.
Gasoline is currently the most important fraction of oil. But in the refining process, a very small percentage (7% to 15%) of gasoline is obtained in relation to current demand. Cracking solves this problem, as it increases the quantity and quality of the gasoline produced. It helps to increase by 20 to 50% the amount of gasoline produced per barrel of oil.
Cracking is carried out in fractionation columns at oil refineries and there are two types: thermal and catalytic cracking. O thermal crackingIt is carried out with high temperature and pressure that break the heavier molecules. For example, in the cracking of kerosene, oil diesel and from the lubricating oil in gasoline, temperatures ranging from 450 to 700ºC are used. already the catalytic cracking it differs from thermal only by the use of a catalyst. You catalysts they are substances capable of increasing the speed of certain chemical reactions without participating in the reaction, that is, they are regenerated at the end of it.
Due to the use of heat, this process is also called pyrolysis, a name that derives from the Greek terms piro, which means "fire", and lysis, which means “break”. Thus, pyrolysis can be defined as "break by fire” and is a type of chemical reaction of decomposition or analysis, in which heat decomposes a substance into two or more products.
Oil cracking is also economically important because many of its by-products are used as raw material in the production of plastics, rubbers and new materials, as shown in the diagram to follow:
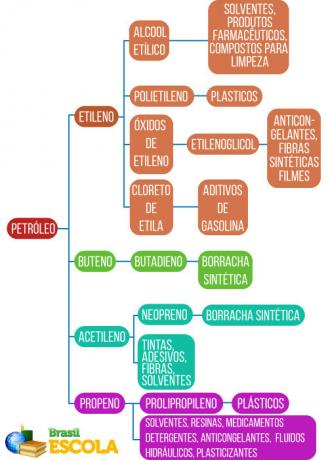
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/craqueamento-petroleo.htm
