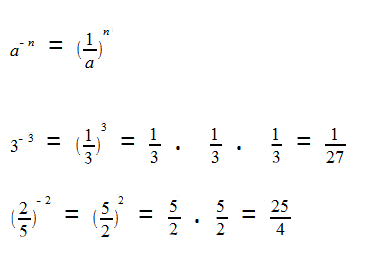The action of a catalyst is basically to accelerate the development of a particular reaction. This is possible because the catalyst changes the mechanism by which the reaction proceeds, leading to an “alternative path” that requires less activation energy for the reaction to start and reach the activated complex.
There are several types of catalysis, one of which is the heterogeneous catalysis, which can be defined as that which occurs when the system has more than one phase, that is, the reactants and products are in a physical state different from the physical state of the catalyst.
An example we can mention is an intermediate stage of sulfuric acid formation (H2ONLY4(aq)). This step consists of the formation of sulfur trioxide (SO3(g)) through the combustion reaction of sulfur dioxide (SO2(g)):
2 SO2(g) + O2(g) → 2 OS3(g)
As this reaction proceeds so slowly, a catalyst is used to accelerate it. A catalyst that can be used in this case is divanadium pentoxide (V2O5(S)), which is solid. Since the reactants and reaction product are gaseous, we will have a heterogeneous system.
But how can divanadium pentoxide speed up the reaction?
What happens is that the molecules of the oxygen reagent are adsorbed, that is, retained on the surface of the divanadium pentoxide. This causes the bonds of the molecules of this gas to weaken over time, which facilitates the formation of the complex activated and, consequently, decreases the activation energy of the reaction, increasing its rate of development, that is, its velocity.
See how this happens in the diagram below:
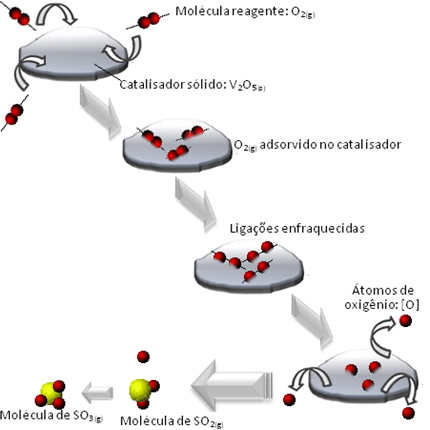
Other examples of heterogeneous catalysis are shown below. Note that in both cases the reactants and products are in the gaseous, aqueous or liquid states, while the catalysts are in the solid state:
An example of heterogeneous catalysis that occurs in our daily lives is that of converters car catalytic, better known as catalysts. These anti-pollution devices are coated with substances that act as catalysts, being usually an alloy of palladium and rhodium (for gasoline engines) and palladium and molybdenum (for gasoline engines). alcohol).
Within this catalyst, chemical reactions occur in which gases from incomplete combustion, which are more harmful to humans, are converted into non-toxic gases. Reactants and products are all gases, whereas catalysts are solids.
To understand more about the operating system of this equipment, read the text: "Catalytic Converter”.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/catalise-heterogenea.htm