Joseph Lous Gay-Lussac (1778-1850) was a scientist who made important studies of gases. He carried out the production of water from the reaction between hydrogen and oxygen gases and verified that they always reacted at the ratio of two volumes of hydrogen to one volume of oxygen, producing two volumes of Water:
Hydrogen + Oxygen → Water
1st Experiment: 2 L 1 L 2 L
2nd Experiment: 4 L 2 L 4 L
3rd Experiment: 8 L 4 L 8 L
4th Experiment: 16 L 8 L 160 L
Note that in all experiments the ratio is always the same: 2: 1: 2.
After several experiments and analyses, realizing that the same occurred with other types of reactions between gases, that is, the reactions always followed a constant volume ratio, this scientist arrived at the following law of reactions in gaseous volumes, known as Gay-Lussac Volumetric Law or Law of Combination of Volumes:

Statement of the volumetric law of Gay-Lussac
But this law was against the Dalton's atomic theory, which said that everything was formed by massive and indivisible spherical particles, the atoms. According to this theory, the volume of the products in the reaction should be equal to the sum of the volumes of the reactants. Thus, the following should occur:
Hydrogen + Oxygen → Water
2 volumes + 1 volume → 3 volumes
But Gay-Lussac showed that this was not the case in practice, the result was equal to two volumes of water vapor.
The answer to this apparent contradiction came through the hypothesis or Avogadro's law.

Stamp printed in Italy shows Amedeo Avogadro and the enunciation of his law, in 1956*
Amedeo Avogadro (1776-1856) showed that, in reality, gases were not isolated atoms, but molecules (with the exception of the noble gases). His law said:

Avogadro's Law Statement
Avogadro showed that 1 mole of any gas has 6.02. 1023 molecules. This value is known as Avogadro's number or constant. It was proven that, in the Normal Conditions of Temperature and Pressure (CNTP), in which the pressure is equal to 1 atm and the temperature is 273 K (0°C), the volume occupied by 1 mole of any gas will always be 22.4 L. This value corresponds to the molar volume of gases. These relationships are very important for solving exercises of stoichiometry.
This may seem strange, as the following question may arise: How could gases with molecules and atoms of different sizes occupy the same volume?
Well, this is because the gas molecules are so far apart that the size of the molecules is negligible.
In this way, Avogadro's volumetric law explained Gay-Lussac's volumetric law. Note below that two hydrogen molecules (two volumes) react with one oxygen molecule (one volume) to form two water molecules (two volumes). Water and hydrogen have the same volume because they have the same amount of molecules, as stated by Avogadro's law.
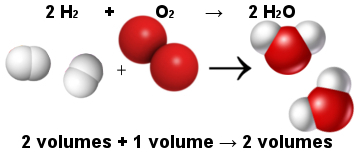
Proportion between molecules in the water formation reaction
At the same time, Avogadro's law made the atomic theory stand, as you see that in both reactants and products there are a total of 6 atoms (4 hydrogen and 2 oxygen).
These volumetric laws were very important for the development of the concept of molecules.
* Image copyrighted: rook76 / Shutterstock.com
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/lei-volumetrica-gay-lussac.htm

