Acetones belong to the class of Ketones (organic oxygenated substances characterized by having the carbonyl in secondary carbon). They are also called propanones or dimethyl ketones, and have general formula C3H6O. Physical aspects: colorless liquid, with a pleasant smell and miscible with water.
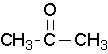
Acetone structural formula
Acetones are industrially obtained through different processes, one of them is through secondary alcohols in a process known as dehydrogenation. Follow the process:

Note that through the oxidation of a secondary alcohol it was possible to obtain propanone.
Acetone Uses
Acetone has wide application in the market, let's see some:
• Solvent for paints, varnishes and enamels;
• Used in the preparation of artificial silks, celluloid and dyes;
• Used to obtain medicinal products (chloroform, bromoform and iodoform).
By Líria Alves
Graduated in Chemistry
Brazil School Team
See more!Ketones
Organic Functions - Organic chemistry
Chemistry - Brazil School

