O melting and boiling point are, respectively, the temperatures at which materials change from solid to liquid and from liquid. for gas or the maximum temperature at which the liquid can remain in this physical state in a given pressure.
The melting and boiling points of the chemical elements on the Periodic Table vary according to their atomic numbers, which then means that they are periodic properties.
In the Periodic Table, the order of growth of the melting and boiling temperatures of the chemical elements follows the following arrow scheme:
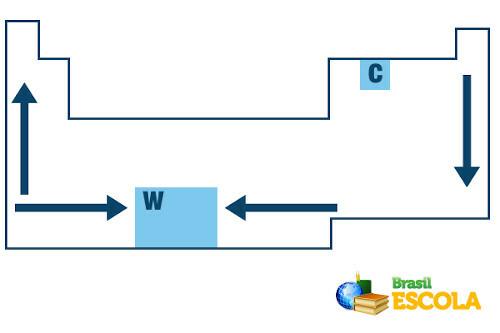
Melting and boiling point growth in the Periodic Table
Note that when we consider the elements belonging to the same family on the left side of the Table, the melting and boiling points decrease as the element's atomic number increases, that is, from below to up. This can be seen in the melting and boiling point values at 1 atm for the family 1 elements shown below:

Melting and boiling points of family 1 elements
On the right side of the Periodic Table, the opposite occurs, the direction of growth of the melting and boiling point of the elements belonging to the same family increases from top to bottom. Therefore, the elements with the lowest melting and boiling temperatures are located at the top of the Table. The only exception is carbon, which has a melting point of 3550 °C and a boiling point of 4287 °C.
Otherwise, most of those with low melting and boiling points are either gases or liquids at room temperature at sea level. As is the case of noble gases, nitrogen, oxygen, fluorine and chlorine, which are in the upper right part of the table.
Now, when it comes to elements belonging to the same period (same row in the Table), we see that the melting and boiling points increase from the sides to the center of the Table. See the example for the elements of the second period:
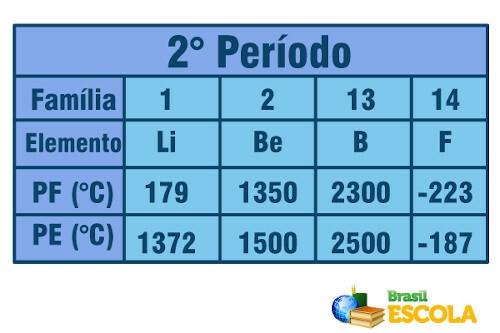
Melting point and boiling point for the elements of the second period of the Periodic Table
Tungsten (W) is an element that is in the center of the Periodic Table and its melting point is the highest among metals, being equal to 3422ºC. That's why it is used in incandescent light bulb filaments, as it can withstand high temperatures without melting.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/ponto-fusao-ebulicaopropriedades-periodicas.htm