Thalidomide is a drug that became widely marketed in Europe, in the 50s and 60s, as a sedative to relieve nausea in pregnant women. It was made in the form of its racemic mixture, that is, as can be seen below, the molecule of this compound is asymmetric, as it has a chiral carbon (carbon with the four different ligands each other). This means that thalidomide has two space isomers or stereoisomers, which are better called enantiomers, since they are the mirror image of each other:

Thalidomide enantiomers have optical activity. dextrorotatory or enantiomer (R) shifts the plane of polarized light to the right, and the levorotatory or enantiomer (S) shifts the plane of polarized light to the left. Thus, a racemic mixture is optically inactive because it contains equal parts of these two enantiomers.
However, this difference in the spatial conformation of atoms ends up resulting in different biological properties, that is, different activities carried out in a living organism. Over time, it was discovered that
only the right-hand isomer or (R) it was responsible for the analgesic, sedative and before-feeling properties, while thalidomide levogira (S) it is teratogenic, that is, it causes mutations in the fetus.For this reason, during the mentioned decades around 12 thousand children were born with malformations. Among the side effects that thalidomide can cause in fetuses are: incomplete development or defective limbs, malformation of the heart (such as the absence of atria), bowel, uterus and gallbladder biliary; effects on the muscles of the eyes and face, deafness, defects in the tibia and femur, and, as the following image shows, thumb with three joints:
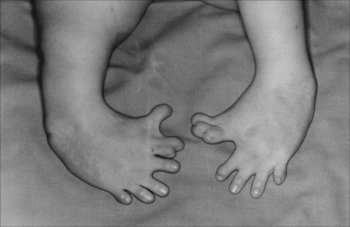
Congenital malformation of the feet, one of the effects of using thalidomide on fetuses*
the este u tion about the chirality of thalidomide, as well as that of other medications, is increasing. Currently, thalidomide is banned from use in pregnant women and in women who are not using two contraceptive methods under strict medical supervision.
It is important to emphasize that even when the drug has only the (R) enantiomer, it still leads to malformation of fetuses because, in the organism, the dextrorotatory isomer undergoes racemization and originates the levorotatory isomer, which is the teratogenic.
Furthermore, the danger still exists because thalidomide is a very effective drug against to other diseases, such as leprosy. Therefore, Anvisa (National Health Surveillance Agency), at Resolution of the Collegiate Board - RDC/Anvisa No. 11, of March 22, 2011, provides for the control of the substance and the drug Thalidomide. Throughout 2013, The Anvisapromoted training courses in order to guide the professionals involved in relation to the control of the drug Thalidomide, reinforcing the risk inherent to this substance, discuss and clarify doubts about the subject, in addition to preventing the deviation of this drug.
* Image credit: Author:Otis Historical Archives National Museum of Health and Medicine/ Source: Wikimedia Commons.
By Jennifer Fogaça
Graduated in Chemistry